ONLINE INQUIRY

Human Epidermal Keratinocytes-adult (HEK-a)
Cat.No.: CSC-7791W
Species: Human
Source: Epidermis; Skin
Cell Type: Keratinocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human epidermal keratinocytes-adult (HEK-a) account for more than 90% of the epidermis, the physical outermost skin layer, and are involved in native immune defence. Keratinocytes are the keratinised, stratified squamous epithelium. They are the cells that undergo various differentiation or keratinisation processes within the epidermis, ascending from the basal layer into the spinous, granular and cornified layers. Regardless of their location in each layer, the shape and function of keratinocytes are controlled by highly regulated processes. Transcriptors including p63 and AP-2, as well as Notch and Wnt pathways, control keratinocyte proliferation, differentiation and survival.
Such cells can recognise cytokines and pathogen-specific molecular patterns. HEK activate inflammatory cells by activating pro-inflammatory cytokines and chemokines, cytokine receptors and microbial probes, such as Toll-like receptors (TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR9), MDA5 (melanoma differentiation-associated gene 5), and RIG-I (retinoic acid-inducible gene I). HEK, in turn, induces antimicrobial activity through release of antimicrobial peptides including defensins, cathelicidins and S100 proteins. By way of these immune processes, HEK contributes to inflammatory skin disorders such as atopic dermatitis (AD), psoriasis and pustular diseases. Therefore, HEK-a is often used to model the development and progression of skin diseases, and help to develop a detailed understanding of how disease works and how to treat it. Additionally, as signs of aging gradually appear in the skin with advancing age, HEK-a are frequently used to screen anti-aging drugs to evaluate their effect on improving skin aging.
 Fig. 1. Primary culture of normal human epidermal keratinocytes (Barthe M ,Jean-Paul Thénot, et al., 2020).
Fig. 1. Primary culture of normal human epidermal keratinocytes (Barthe M ,Jean-Paul Thénot, et al., 2020).
TRPV3 Stimulation Suppresses Proliferation of Epidermal Keratinocytes and Induces Cell Death
The functions of TRP channels were initially focused on sensory neurons, primarily linked to sensory functions such as pain, itch, thermal hyperalgesia, thermosensation, and mechanosensation. Recent decades have seen research indicating the presence of TRP channels in non-neuronal cells, especially their functions in the skin. As part of the TRP channel family, TRPV3 was first discovered in keratinocytes. Research indicates that TRPV3 modulates skin health, including the epidermal barrier, hair production, and melanogenesis. Szöllősi et al. further explored the innate function of TRPV3 in human normal epidermal keratinocytes (NHEKs) to clarify how it affects the most common skin cell at the cellular level.
They tested the cell-level consequences of TRPV3 activation on the growth and survival of NHEKs by subjecting them to different TRPV3 agonists including carvacrol (a plant derivative) and the synthetic agonist 2-APB. The results demonstrated that both carvacrol and 2-APB reduced cell viability and proliferation of NHEK cells with increasing doses (Fig. 1a and b). Carvacrol and 2-APB activity were confirmed via RNAi to be TRPV3 specific. TRPV3 silencing nearly eradicated the damaging effects on cell survival and growth, which imply TRPV3's involvement in these cellular functions. Concentrations of carvacrol and 2-APB substantially reduced mitochondrial membrane potential (an early apoptotic indicator) but not membrane integrity, indicating apoptotic cell death (Fig. 2a). EDTA did not alter these results, indicating that the effect relies on calcium mobilization from the extracellular space (Fig. 2b). As shown above, stimulation of TRPV3 decreases the proliferation and death of human epidermal keratinocytes.
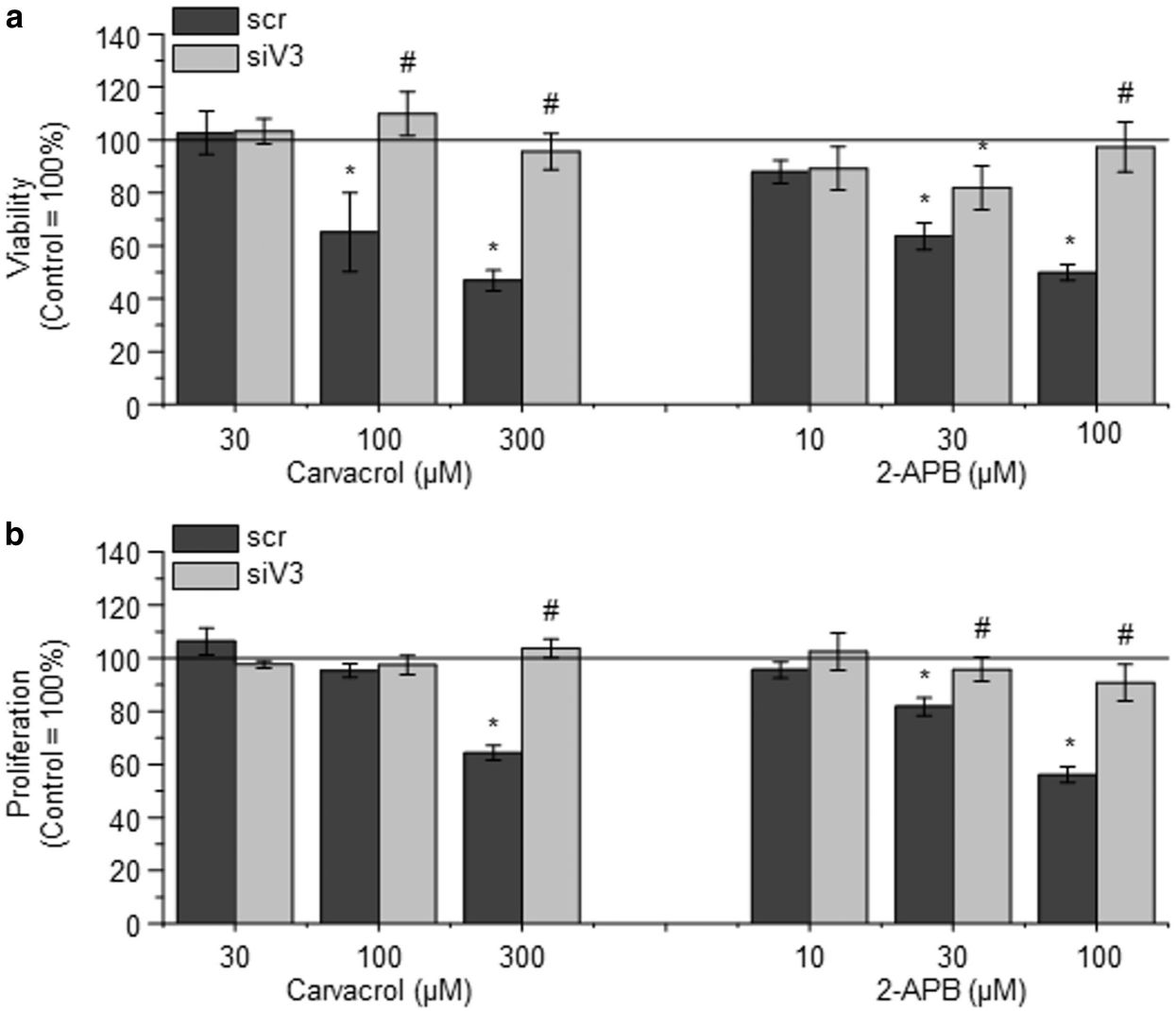 Fig. 1. Activation of TRPV3 on normal human epidermal keratinocytes (NHEKs) decreases viability and inhibits proliferation (Szöllősi AG, Vasas N, et al., 2018).
Fig. 1. Activation of TRPV3 on normal human epidermal keratinocytes (NHEKs) decreases viability and inhibits proliferation (Szöllősi AG, Vasas N, et al., 2018).
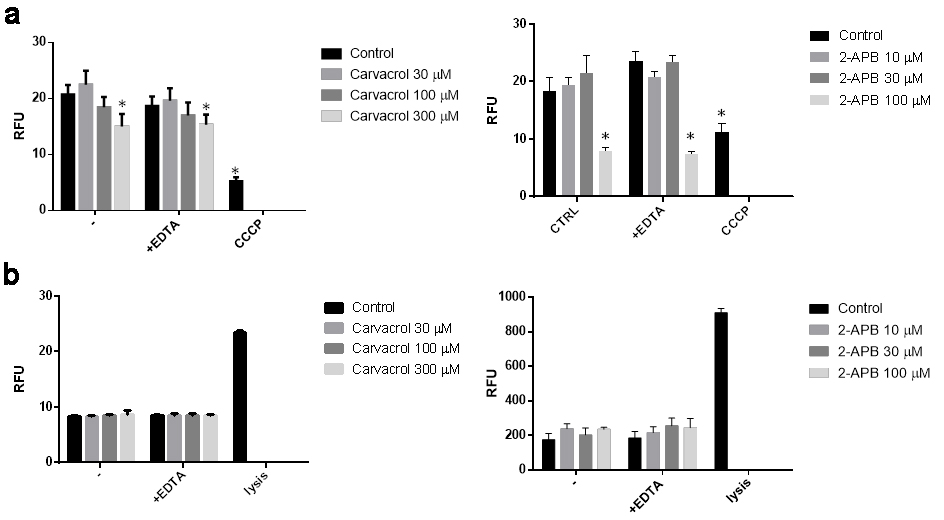 Fig. 2. Activation of transient receptor potential vanilloid-3 (TRPV3) on normal human epidermal keratinocytes (NHEK) causes apoptosis (a) and does not cause necrosis (b) (Szöllősi AG, Vasas N, et al., 2018).
Fig. 2. Activation of transient receptor potential vanilloid-3 (TRPV3) on normal human epidermal keratinocytes (NHEK) causes apoptosis (a) and does not cause necrosis (b) (Szöllősi AG, Vasas N, et al., 2018).
6-Shogaol Prevents UVB Radiation Mediated Inflammation and Oxidative Stress in Human Epidermal Keratinocytes
The skin can defend itself against external attack, but chronic exposure to UV (ultraviolet) rays promotes inflammation, photoaging and nonmelanoma skin cancers. Additionally, UVB exposures promote reactive oxygen species (ROS) and oxidative stress. Oxidative stress defences are mediated by the nuclear factor erythroid 2-related factor 2 (Nrf2). 6-Shogaol (a ginger-based extract) has anti-inflammatory and antioxidative properties. Although it induces Nrf2 signalling in HEK293 and HepG2 cells, it does not appear to function in human epidermal keratinocytes. Looking at ROS, Nrf2 and MAPK signalling pathways, Chen's team examined how 6-shogaol functioned in the body.
ROS and inflammation markers (COX-2 and iNOS) tests showed that 6-shogaol significantly inhibited ROS production and COX-2 and iNOS expression, suggesting that it suppressed UVB-induced inflammation in keratinocytes. UVB rays create ROS, activating MAPK signals and causing oxidative stress. In order to explain the molecular mechanism, they looked at the MAPK family in UVB-treated keratinocytes. The results showed that UVB increased p38, ERK, and JNK levels (Fig. 3A and B). In contrast, 20 μg of 6-shogaol significantly reduced their phosphorylation compared to UVB-only cells. Western blot analysis confirmed that 6-shogaol inhibits UVB-induced overexpression of these proteins (Fig. 3D). Additionally, UVB exposure decreased Nrf2 levels, but 6-shogaol prevented this reduction. UVB (180 mJ/cm2) significantly lowered Nrf2 protein levels, while 6-shogaol preserved them and increased HO-1 levels in UVB-irradiated cells (Fig. 4A). RT-PCR results for Nrf2 and HO-1 mRNA expression confirmed these findings (Fig. 4B). Overall, 6-shogaol may serve as an effective agent to enhance resistance against UVB-induced inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes.
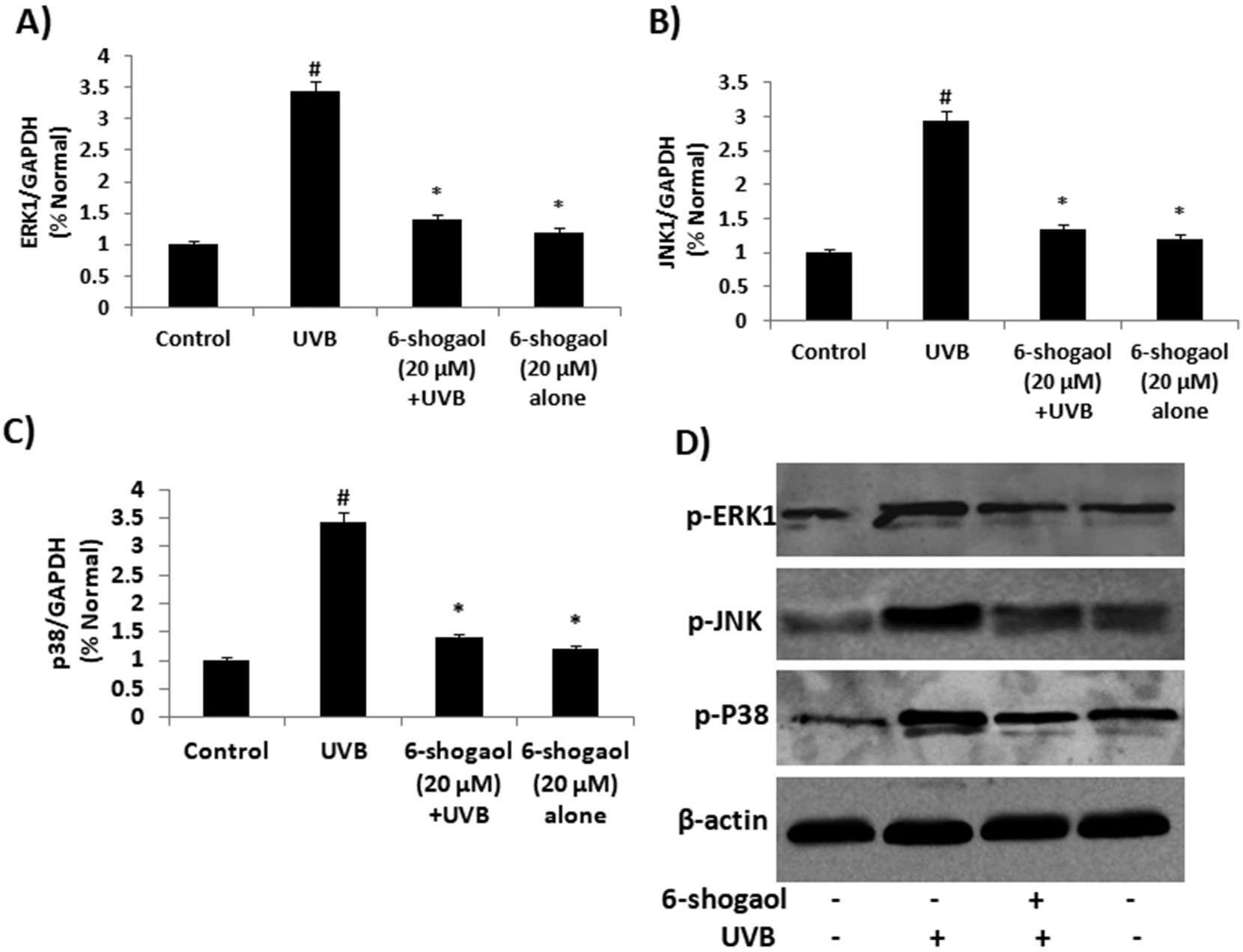 Fig. 3. 6-shogaol on UVB induced MAPKs signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
Fig. 3. 6-shogaol on UVB induced MAPKs signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
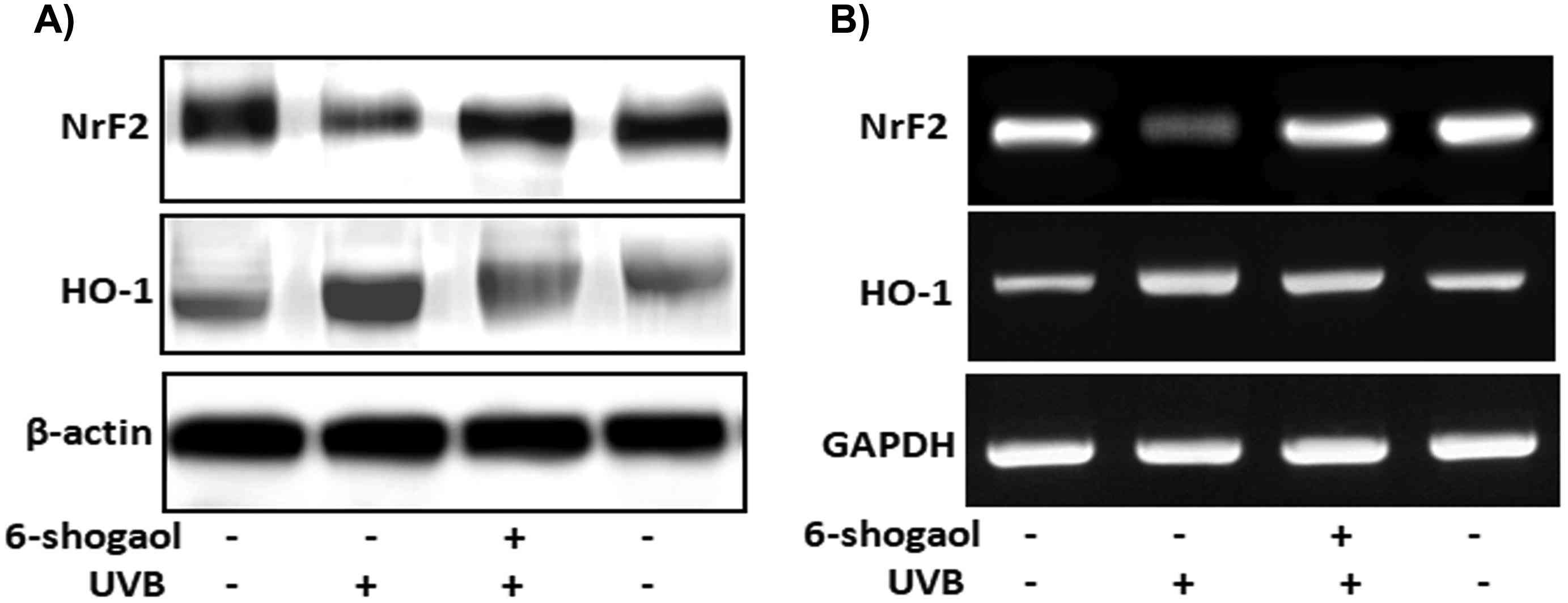 Fig. 4. 6-shogaol on UVB induced NrF2 signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
Fig. 4. 6-shogaol on UVB induced NrF2 signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
Ask a Question
Write your own review