ONLINE INQUIRY

Human Dermal Microvascular Endothelial Cells
Cat.No.: CSC-C8610W
Species: Human
Source: Dermis; Skin
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The human dermal microvascular endothelial cells (HDMECs) are a monolayer of flat cells lining the inside walls of dermal microvessels that is arranged in a polygonal or cobblestone shape. A part of the vascular endothelium, they regulate angiogenesis, substance exchange, and coagulation.
These cells form a smooth inner surface of the vascular body that provides a high-throughput blood supply, while also interfering with the basement membrane to form a selectively porous membrane that controls the ingress of fluids, gases and macromolecules through the vessel walls. HDMECs produce and secrete a variety of bioactive molecules such as prostacyclin, nitric oxide, and endothelin to regulate normal vascular function. They play an essential role in inflammatory response, tumorigenesis and wound repair, and contribute to physiology through the maintenance of hemostasis, heat regulation and the trafficking of leukocytes. During wound healing, they facilitate repair and remodelling of microvascular structures by regulating cell processes including proliferation and apoptosis. In the laboratory, these cells are primarily used to investigate the microvascular architecture and disease mechanisms of skin. They are, for example, examined in hypoxia post-conditioning mice to evaluate their ischemia-reperfusion injury protective potential. HDMECs are also used for drug screening and toxicity testing, which measure their response to medications.
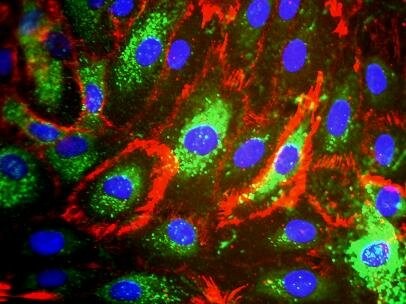 Fig. 1. Phenotypic characteristics of Human dermal microvascular endothelial cells (Agarwal S, Das H, et al., 2010).
Fig. 1. Phenotypic characteristics of Human dermal microvascular endothelial cells (Agarwal S, Das H, et al., 2010).
Effects of DA-9801 on the Inflammatory Stress and Apoptosis Induced by Ang II in HDMECs
Angiotensin II (Ang II) causes vascular damage by activating reactive oxygen species (ROS), activating proinflammatory genes, and killing endothelial cells by activating the type I Ang II receptor. DA-9801, a plant-based treatment for diabetic neuropathy, has been shown to reverse angiotensin II (Ang II)-mediated vascular endothelial cell damage. But the mechanism remains unclear. Hong's team aims to explore whether DA-9801 counteracts Ang II-induced endothelial cell dysfunction by reducing inflammation and apoptosis in human dermal microvascular endothelial cells (HDMECs).
Changed expression of eNOS and iNOS are associated with Ang II cytotoxicity. Hence Hong's group investigated whether Ang II damages cells by altering HDMEC eNOS and iNOS levels. iNOS was undetectable in HDMECs left without treatment, and Ang II therapy raised iNOS and reduced eNOS (Fig. 1A). DA-9801 prevented the Ang II-induced decrease in extracellular NO, but DA-9801 alone didn't alter NO levels (Fig. 1B). Monocytes stick to the endothelium through the actions of adhesion molecules such as ICAM-1, VCAM-1 and E-selectin. Ang II raised HDMEC ICAM-1, VCAM-1, and E-selectin levels while DA-9801 decreased them (Fig. 1C). To determine whether Ang II induces death via caspase-mediated pathways, Ang II treated cells were analysed with TUNEL and annexin V assays. Caspase-3, a critical mediator in mitochondrial and death receptor apoptosis, is stimulated from its precursor by initiator caspases. Here, DA-9801 inhibited Ang II-mediated caspase-3 degradation and initiater caspase-9 and 7 productions (Fig. 2A and B). Ang II-treated cells had greater green fluorescence and condensed nuclei (indicating apoptosis) compared with DA-9801 treated cells (Fig. 2C and D). In this way, DA-9801 controlled the pathology of Ang II-induced endothelial cell dysfunction through inflammatory and apoptotic pathways.
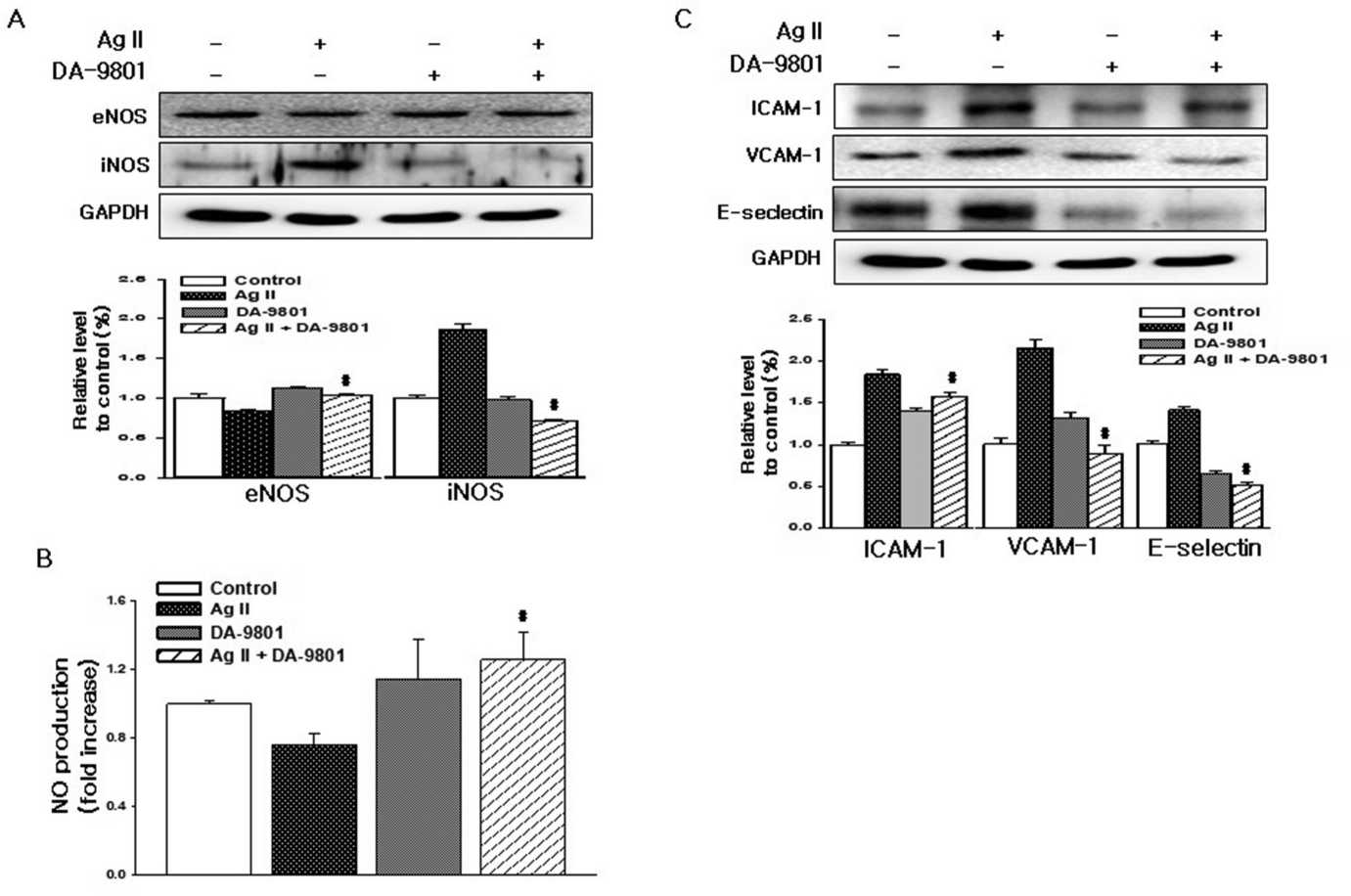 Fig. 1. DA-9801 inhibits Ang II-induced inflammatory stress in HDMECs (Hong OK, Lee SS, et al., 2021).
Fig. 1. DA-9801 inhibits Ang II-induced inflammatory stress in HDMECs (Hong OK, Lee SS, et al., 2021).
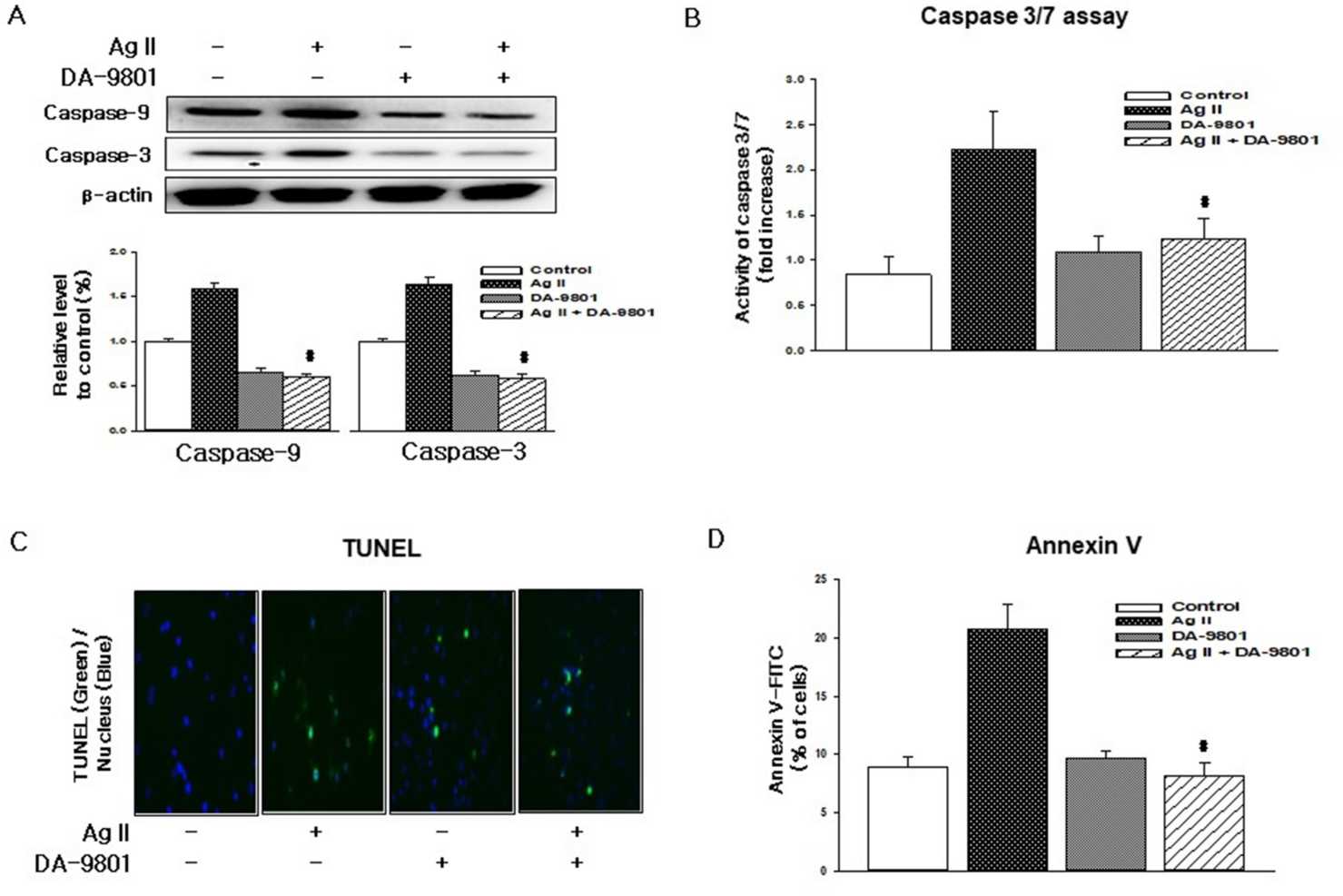 Fig. 2. DA-9801 inhibits Ang II-induced apoptosis in HDMECs (Hong OK, Lee SS, et al., 2021).
Fig. 2. DA-9801 inhibits Ang II-induced apoptosis in HDMECs (Hong OK, Lee SS, et al., 2021).
MPS strengthens HDMECs and HPCs interactions through Ang-1/Tie2 Pathway Activation
Skin homeostasis depends on dermal microvessels for essential supplies, but aging disrupts vascular structure, leading to diminished capillary density and microvascular abnormalities in diseases like psoriasis and diabetic retinopathy. Mucopolysaccharide polysulfate (MPS) is a heparinoid and MPS-containing formulations are widely used as moisturizers for dry skin and to treat peripheral vascular insufficiency. Although MPS has curative applications in skin conditions where microvascular abnormalities occur, the mechanisms by which MPS influences microvascular function are incompletely known. In vitro, Fujiwara-Sumiyoshi's team measured the functional properties of MPS on human pericytes (HPC) and human dermal microvascular endothelial cells (HDMEC), as well as on skin microvascular permeability.
The Ang-1/Tie2 signalling system plays an important role in maintaining the integrity of the blood vessels; Ang-1 improves the endothelial barrier to keep the blood from leaking out. Fujiwara-Sumiyoshi et al. found that MPS (>1 g/mL) significantly enhanced Ang-1 production in HPC as a function of concentration (Fig. 3a). Moreover, MPS at 10 μg/mL notably enhanced PDGF-BB production in HDMEC compared to the growth medium alone (Fig. 3e). Therefore, MPS boosts Ang-1 production in HPC and PDGF-BB production in HDMEC. Thus, MPS improves HPC Ang-1 production and HDMEC PDGF-BB production. They then examined whether MPS increased expression and phosphorylation of the Ang-1 receptor Tie2 in HDMECs. The results revealed that MPS 0.1 g/mL and 1 g/mL elevated Tie2 levels (Fig. 4a) and markedly increased HDMECs' Tie2 phosphorylation (Fig. 4b and c). These results suggest that MPS potentiates interactions between dermal microvascular endothelial cells and pericytes to enhance microvascular stabilization via activation of the Ang-1/Tie2 signaling pathway.
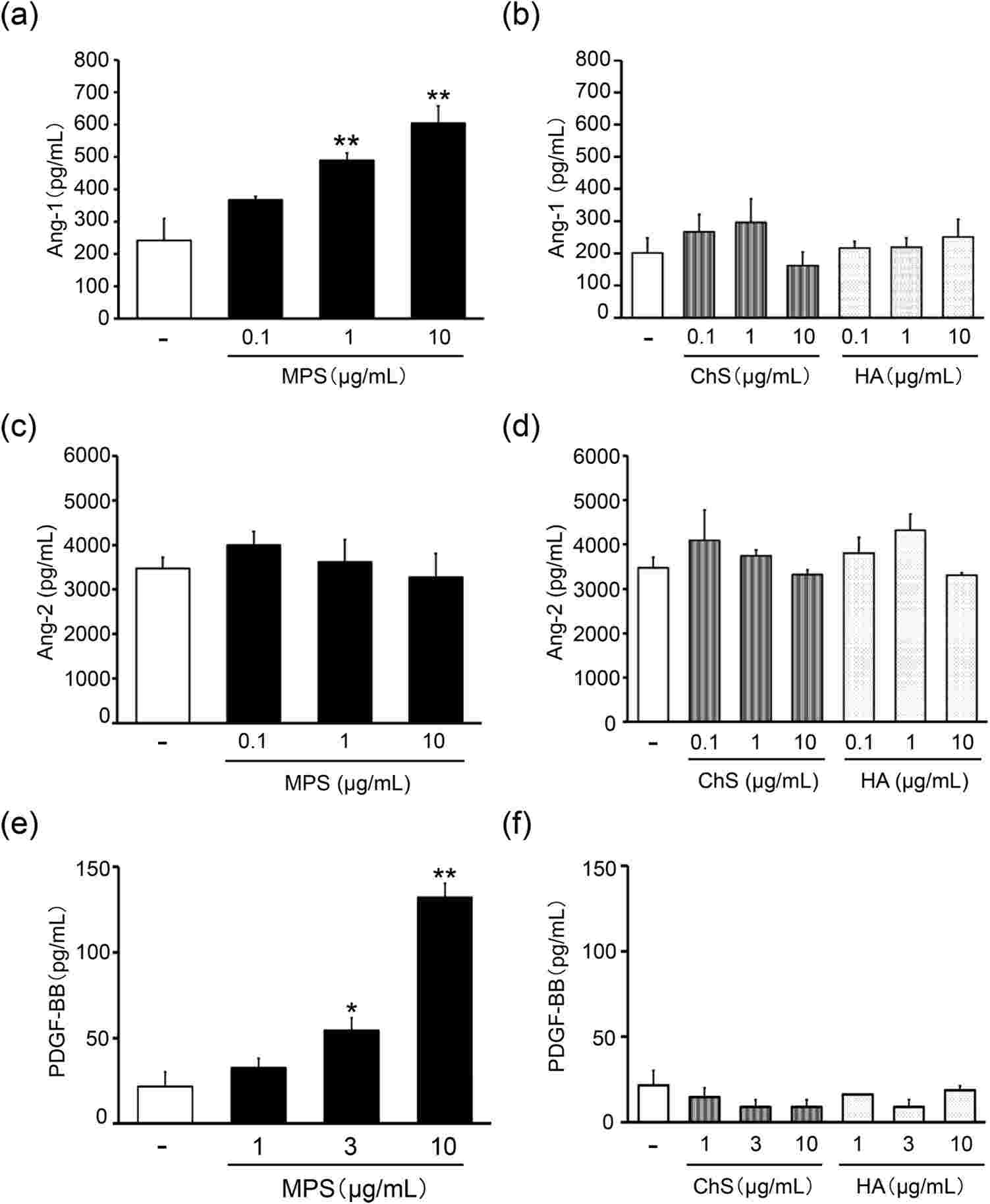 Fig. 3. Effects of MPS on the protein expression of Ang-1 in HPC, and Ang-2 and PDGF-BB in HDMEC (Fujiwara-Sumiyoshi S, Ueda Y, et al., 2021).
Fig. 3. Effects of MPS on the protein expression of Ang-1 in HPC, and Ang-2 and PDGF-BB in HDMEC (Fujiwara-Sumiyoshi S, Ueda Y, et al., 2021).
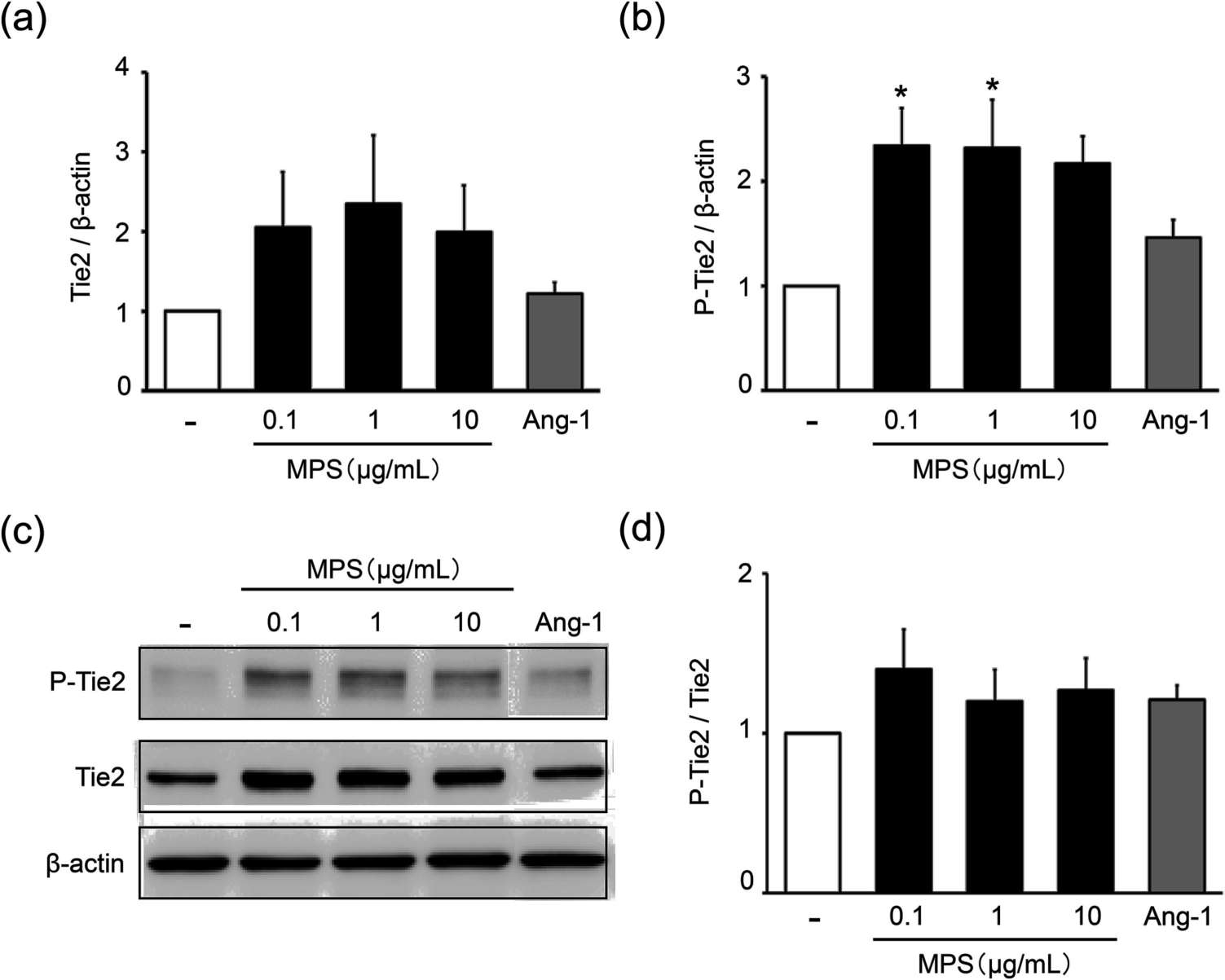 Fig. 4. Effects of MPS on the protein expression of Tie2 and phosphorylated Tie2 in HDMEC (Fujiwara-Sumiyoshi S, Ueda Y, et al., 2021).
Fig. 4. Effects of MPS on the protein expression of Tie2 and phosphorylated Tie2 in HDMEC (Fujiwara-Sumiyoshi S, Ueda Y, et al., 2021).
Ask a Question
Write your own review