ONLINE INQUIRY

Human Dermal Lymphatic Endothelial Cells
Cat.No.: CSC-C8596W
Species: Human
Source: Dermis; Skin
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human dermal lymphatic endothelial cells (HDLECs) are a type of monolayer flat epithelium that are tightly arranged on the inner surface of lymphatic vessels, forming the primary structure of the lymphatic vessel wall. Through their precise arrangement and structure, they ensure effective lymph transportation and the normal functioning of the lymphatic system. HDLECs typically lack a complete basement membrane, which endows them with high permeability, favoring the exchange of substances between lymphatic fluid and tissue fluid. Moreover, HDLECs possess numerous invaginations and cytoplasmic vesicles, along with characteristic overlapping intercellular junctions. These structural features enhance the strength of intercellular connections and help maintain the integrity and stability of lymphatic vessels. Additionally, HDLECs exhibit positive reactions to specific immunofluorescence markers such as CD31, Podoplanin, and Lyve1, which are commonly used for the identification and isolation of these cells.
HDLECs play a vital role in maintaining physiological balance and immune functionality within the human body. They not only manage fluid, protein and tissue pressure but also serve as sites for lymphocyte recirculation and immune activity. Lymphocytes travel efficiently throughout the body, performing immune functions such as infection prevention and tumour surveillance via lymphatic vessels. Moreover, HDLECs are involved in various pathologic processes including wound healing, lymphoedema, and spreading of inflammation. Several new studies have shown that HDLECs play a pivotal role in tumor metastasis, modulating the spread routes of cancer cells through control of the creation and function of lymphatic vessels In research, HDLECs are often used in making models of lymphatic diseases, including lymphangioma, lymphangitis and lymphatic tuberculosis, in order to understand how these conditions can be treated. Besides these, HDLECs are widely applied to determine how lymphatic endothelial cells multiply, migrate and differentiate, and the molecular signalling networks controlling those functions.
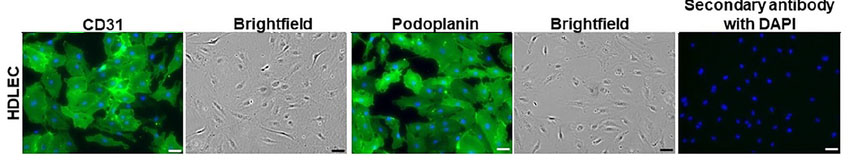 Fig. 1. Human dermal lymphatic endothelial cells. DAPI: blue nuclei. Corresponding bright field images of CD31 and podoplanin immuno-stained cells appear to the right of each immuno-stained image. (Kong AM, Lim SY, et al., 2022).
Fig. 1. Human dermal lymphatic endothelial cells. DAPI: blue nuclei. Corresponding bright field images of CD31 and podoplanin immuno-stained cells appear to the right of each immuno-stained image. (Kong AM, Lim SY, et al., 2022).
Pannexin-1 Modulate In Vitro Lymphangiogenesis
Pannexin-1 (PANX1) belongs to the pannexin family of proteins. It is a transmembrane protein that creates channels that allow ions and small molecules (1 kDa) to move between the extracellular matrix and the cytosol. Although researchers have looked into the vascular system of pannexin PANX1, their research on the development of lymphatic vessels has been limited. Hence, Boucher's team investigated PANX signalling in HDLECs, and more specifically, the role of PANX1 in lymphangiogenesis.
To investigate PANX1's role in lymphangiogenesis, they used an in vitro tube-formation assay to assess LECs' ability to form 3D capillary-like networks on basement membrane extracts (Fig. 1A). Treatment with Probenecid or Brilliant Blue FCF resulted in disorganized networks (Fig. 1A). Tube length decreased by 28 ± 4% and 20 ± 3% with 0.1 and 1 mM Probenecid, and by 21 ± 3% and 29 ± 4% with 1 and 5 µM Brilliant Blue FCF, respectively (Fig. 1B). Junction numbers also decreased by 28 4% and 22 3% with Probenecid, and 25 4% and 33 6% with Brilliant Blue FCF (Fig. 1B). PANX1's contribution was confirmed by the inhibitory peptide 10Panx; administration of 50 or 100 M 10Panx reduced capillary complexes and junctions, respectively, by 22 2% or 34 3% and 25 2% or 40 4% compared with controls (Fig. 1B). In order to verify further the function of PANX1, siRNA was employed to silence its activity in HDLECs; an 80 11% decrease in expression of PANX1 was observed at 24 hours (Fig. 2A). No upregulation of PANX2 or PANX3 was observed (Fig. 2B). In the tube-formation assay, PANX1 siRNA-transfected HDLECs failed to form capillaries (Fig. 2C), with total tube length and junction numbers dropping by 24 4 per cent and 34 3 per cent, respectively (Fig. 2D). These findings reveal that in vitro lymphangiogenesis relies on PANX1.
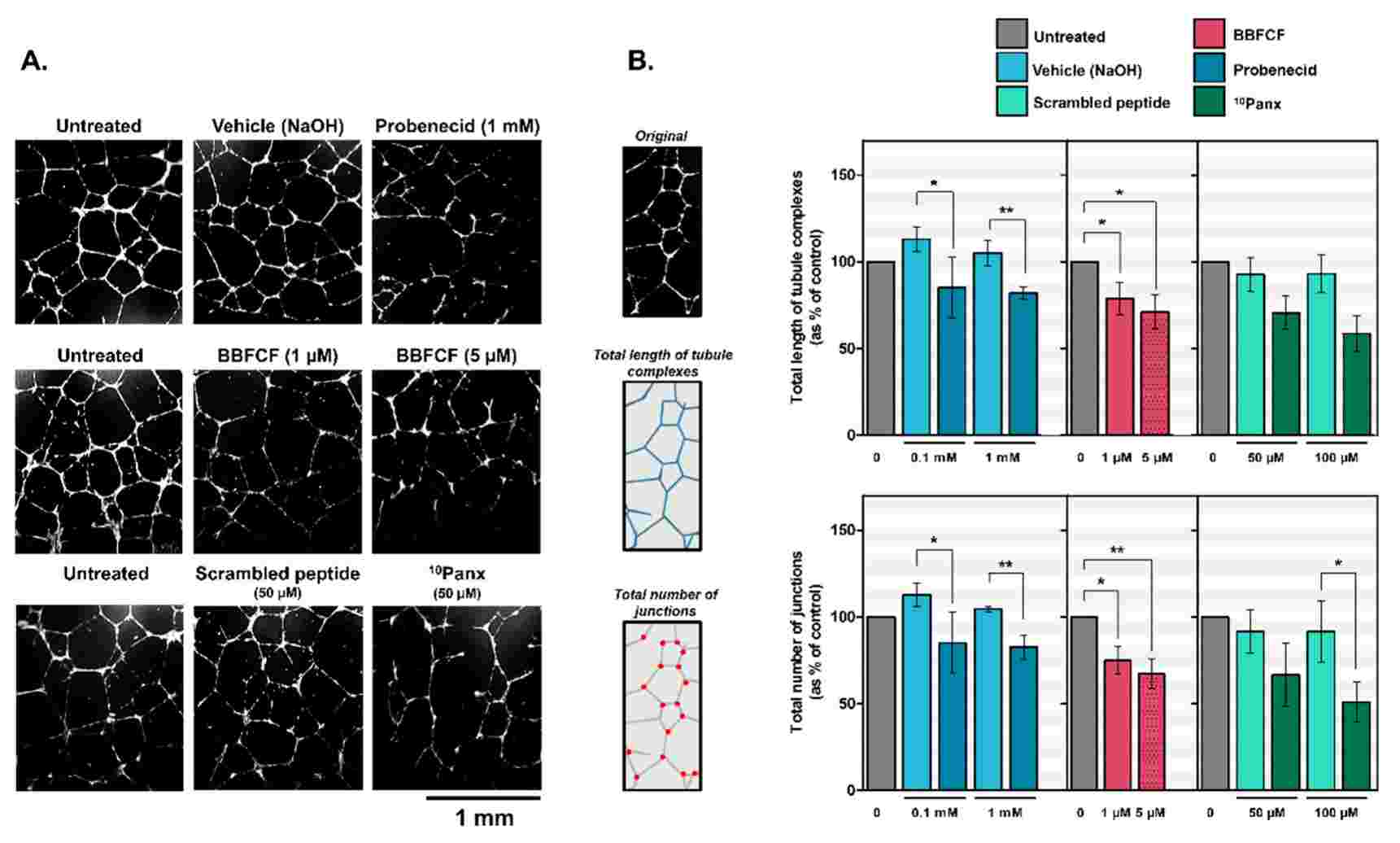 Fig. 1. Inhibition of capillary-like formation in HDLECs by pharmacological inhibitors of Pannexin-1 (Boucher J, Simonneau C, et al., 2018).
Fig. 1. Inhibition of capillary-like formation in HDLECs by pharmacological inhibitors of Pannexin-1 (Boucher J, Simonneau C, et al., 2018).
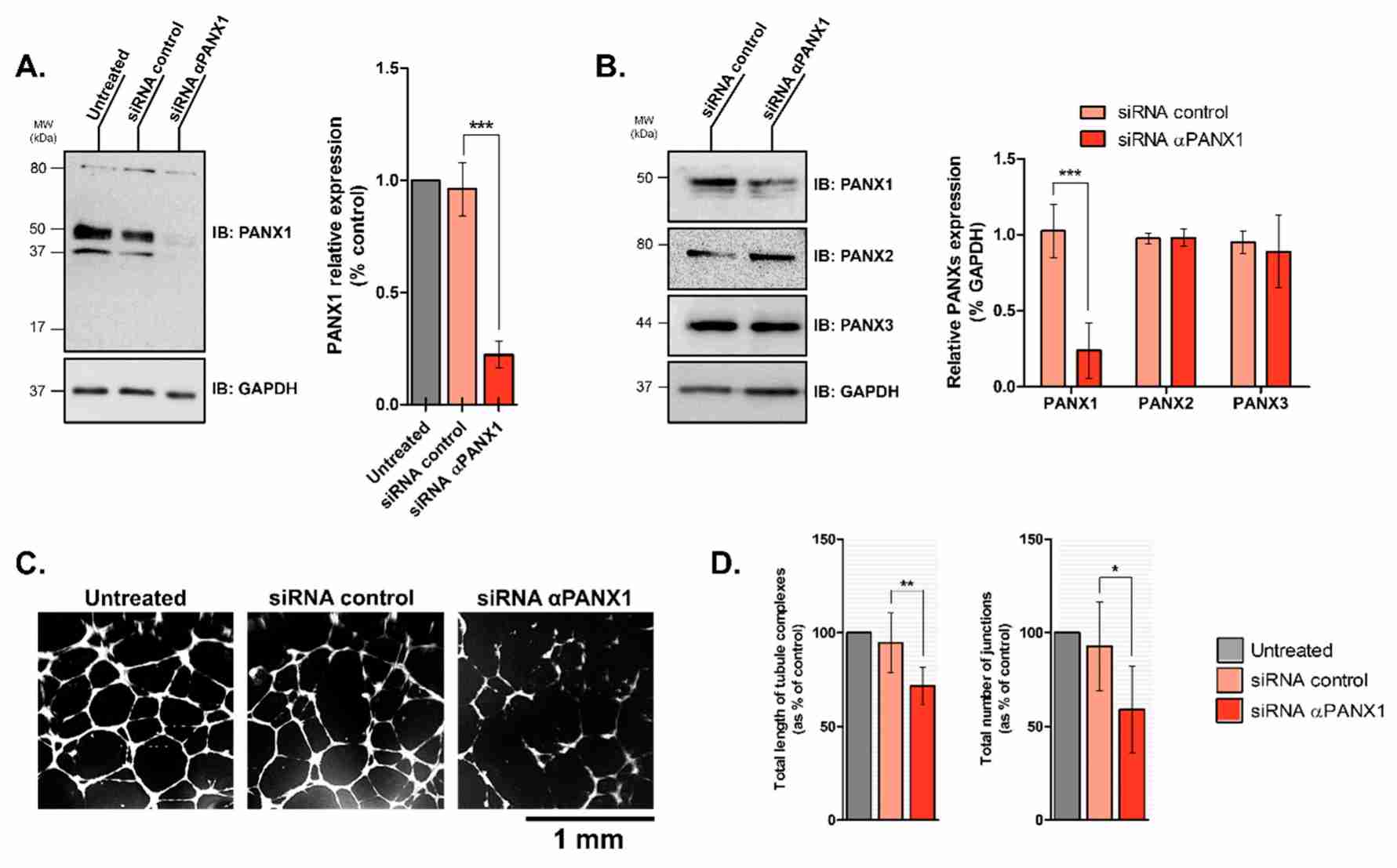 Fig. 2. Silencing Pannexin-1 expression affects capillary-like formation by HDLECs (Boucher J, Simonneau C, et al., 2018).
Fig. 2. Silencing Pannexin-1 expression affects capillary-like formation by HDLECs (Boucher J, Simonneau C, et al., 2018).
VEGF-C Sensitizes Lymphatic Endothelial Cells (LECs) to Oxidative-Stress-Induced Cell Death
Secondary lymphedema arises when the lymphatic tissue is damaged by surgery, cancer treatment, infection, trauma or obesity, creating oxidative stress and hypoxia. Although higher levels of vascular endothelial growth factor C (VEGF-C) may help stimulate lymphangiogenesis, VEGF-C typically doesn't heal the lymphatic tissue damage during lymphedema. And the exact mechanisms underlying lymphedema are not clear.
Hossain's team explored oxidative stress as a cause of lymphedema. The mouse experiments demonstrated that both oxidative stress and VEGF-C deposits in the tail lymphedema model are related to cell death. They then investigated the connection between VEGF-C and cell death in human dermal lymphatic endothelial cells (HDLECs). They injected HDLECs with hydrogen peroxide (H2O2), and compared them with human umbilical vein endothelial cells (HUVECs) as a model for oxidative stress on vascular endothelial cells. They revealed that, in HDLECs and HUVECs, 400 M H2O2 killed 40% of cells (Fig. 3A and B). They tested VEGF-C's function in oxidative-stress death by pre-treatment of HDLECs with 10 ng/mL VEGF-C before exposing them to 500 M H2O2, and saw 45% mortality, as opposed to 25% when they were exposed to H2O2 alone (Fig. 4A). VEGF-C sensitising activity was dose-dependent, with the greatest rise in H2O2-induced death seen at 10 ng/mL (Fig. 4B). VEGF-C and H2O2 both increased cell death to 25% and 55% in HUVECs and rat LECs, respectively (Fig. 4C). These results indicated that VEGF-C at 10 ng/mL predisposes LECs to oxidative-stress-induced cell death and this was then utilized for all subsequent experiments. Moreover, when combined with 2-methoxyestradiol (2-ME) to produce oxidative stress, VEGF-C (10 ng/mL) increased HDLEC cell death from 35 to 45 per cent (Fig. 3C). In HUVECs, VEGF-C and 2-ME increased cell death from 30–40% within 48 hours (Fig. 3D). Thus, both VEGF-C make LEC more vulnerable to oxidative-stress death.
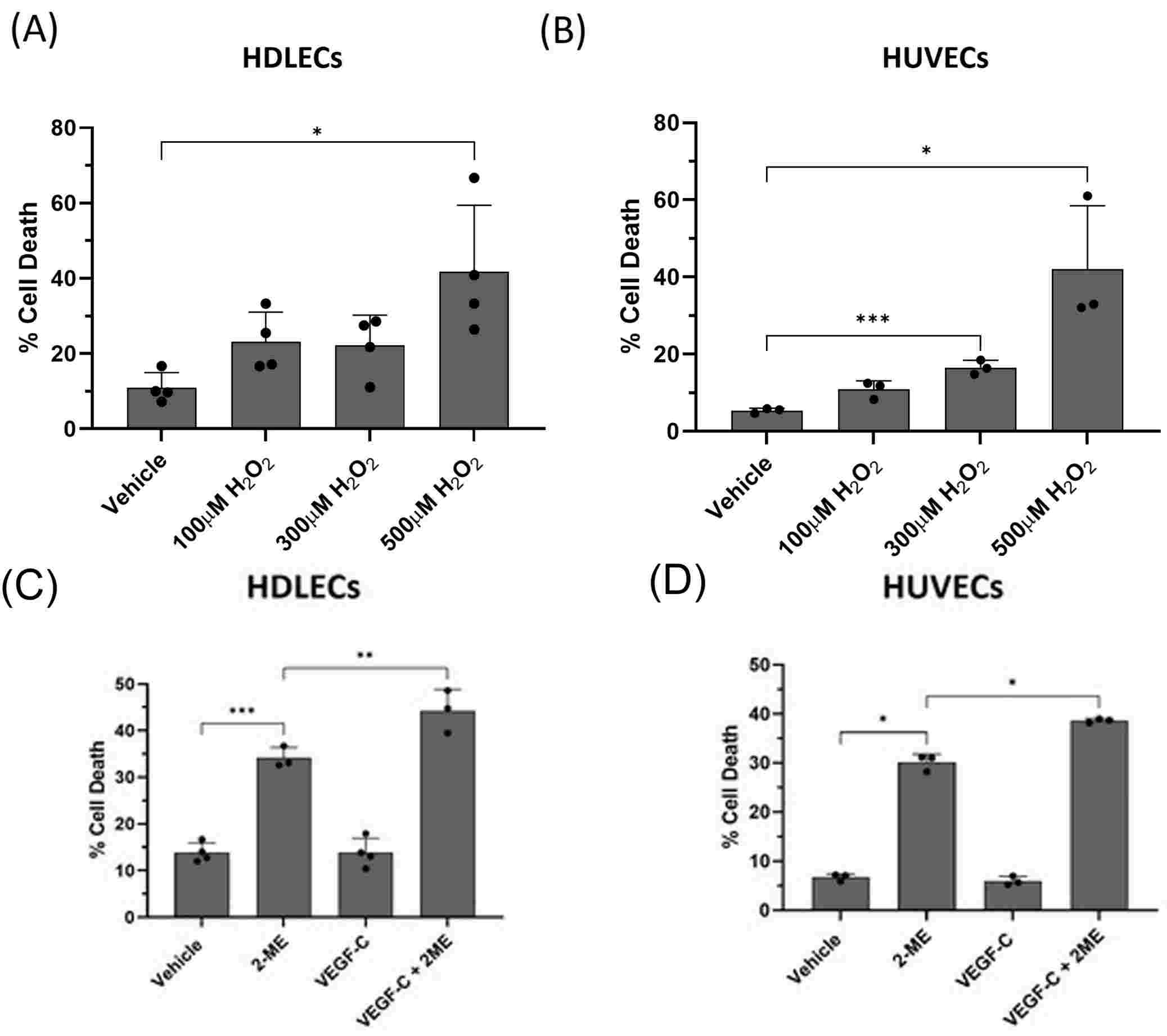 Fig. 3. (A-B). H2O2 causes significant cell death in HDLECs and HUVECs. (C-D). VEGF-C sensitizes HDLECs and HUVECs to 2-ME-induced cell death (Hossain L, Gomes KP, et al., 2024).
Fig. 3. (A-B). H2O2 causes significant cell death in HDLECs and HUVECs. (C-D). VEGF-C sensitizes HDLECs and HUVECs to 2-ME-induced cell death (Hossain L, Gomes KP, et al., 2024).
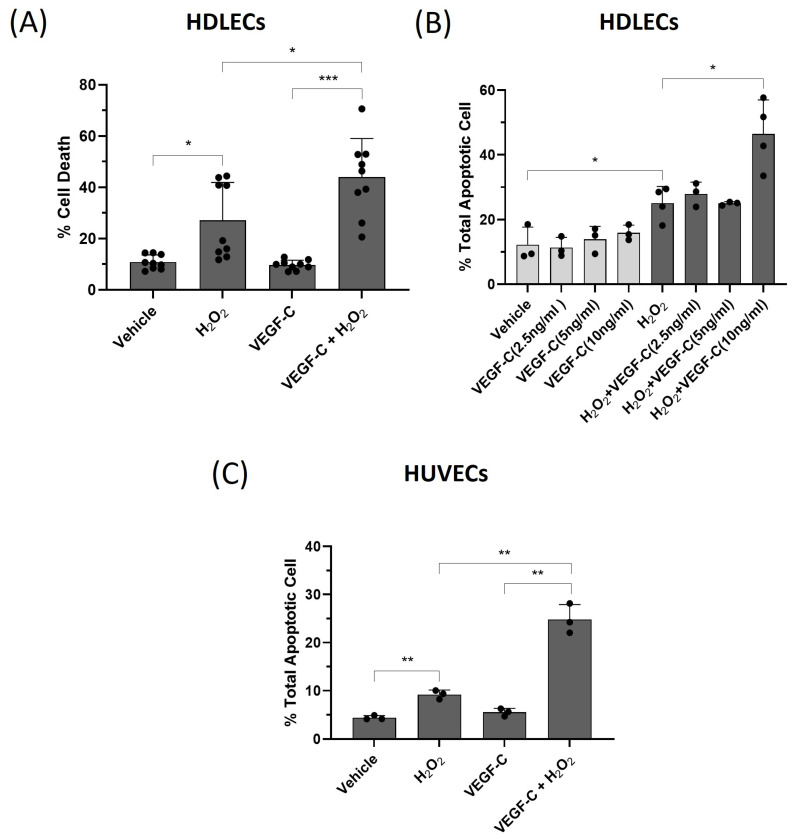 Fig. 4. VEGF-C sensitizes HDLECs to H2O2-induced cell death through apoptosis (Hossain L, Gomes KP, et al., 2024).
Fig. 4. VEGF-C sensitizes HDLECs to H2O2-induced cell death through apoptosis (Hossain L, Gomes KP, et al., 2024).
Ask a Question
Write your own review