ONLINE INQUIRY

Human Dermal Fibroblasts-fetal (HDF-f)
Cat.No.: CSC-7797W
Species: Human
Source: Dermis; Skin
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human dermal fibroblasts- fetal (HDF-f) are isolated from fetal dermal tissue and represent the most prevalent cell type within the loose connective tissue. These cells are typically located in close proximity to collagen fibers, to which they attach. The morphology of the cells is a long spindle with a basophilic granule-rich cytoplasm. Most notably, their surface is packed with high-affinity FcRI receptors that activate free IgE, indicating that they cause type I hypersensitivity responses. HDF-f play a vital role in the formation and development of foetal connective tissue, an essential component of embryonic development. HDF-f have robust proliferative ability and high metabolic activity, so are particularly well suited for in vitro culture and post passaging.
HDF-f is primarily responsible for generating and secreting extracellular matrix components, including collagen and elastin, which help to keep the skin firm, elastic and moist. When skin is damaged, these fibroblasts proliferate and move to the site of the injury where they participate in the repair and regeneration of the wound by producing and releasing matrix materials. This ability is essential for maintaining fetal skin integrity and function. Due to their developmental stage, HDF-f exhibit greater plasticity and possess a distinct gene expression profile compared to adult fibroblasts. Consequently, HDF-f hold significant potential for applications in tissue engineering and regenerative medicine, particularly given their enhanced capacity for regeneration and plasticity.
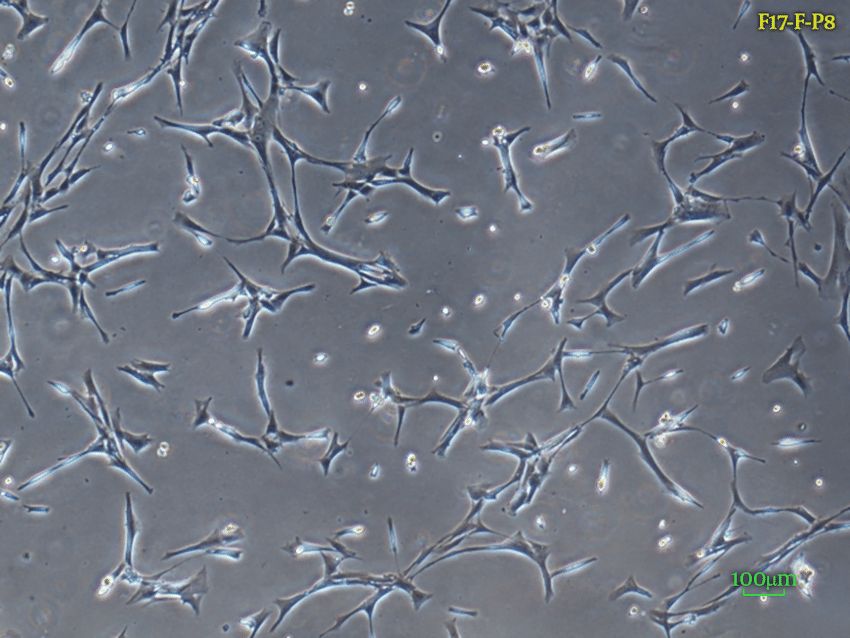 Fig. 1. 40X Inverted light microscopy of fetal dermal fibroblasts morphology (Goodarzi P, Falahzadeh K, et al., 2018).
Fig. 1. 40X Inverted light microscopy of fetal dermal fibroblasts morphology (Goodarzi P, Falahzadeh K, et al., 2018).
hFDFs Exhibit In Vitro Multipotency and Displays an MSC Phenotype.
Developing allogeneic cell therapies that do not depend on large donor areas shows promise in addressing chronic and acute skin wound healing. Human fetal-derived dermal fibroblasts (hFDFs) are of particular interest due to their high division rates, low immunological rejection, and scarless healing ability. Esteban-Vives's team examined the potential of hFDFs as a source for cell banking.
Results indicated that hFDFs share markers and differentiation capabilities with bone marrow-derived mesenchymal stem cells (MSCs), demonstrating therapeutic potential. The hFDF cell populations demonstrated in vitro multipotency, similar to human MSCs. Proteoglycan staining post-induction confirmed osteogenic, adipogenic, and chondrogenic differentiation (Fig. 1A). hFDFs from six donors were cultured up to three passages, showing a decrease in CD105/CD90/CD73 expression from 57% to 15%, and an increase in CD34/CD45/CD14 expression from 0.1% to 2.6% by passage 3, maintaining CD79a/HLA-DR below 0.1% (Fig. 1B). Gene expression analysis from passages 4 to 7 corroborated these trends (Fig. 1C). Although hFDFs showed no significant growth rate differences compared to MSC-sorted hFDFs, their division rate was higher than BM-MSCs, suggesting suitability for cell banking (Fig. 1D).
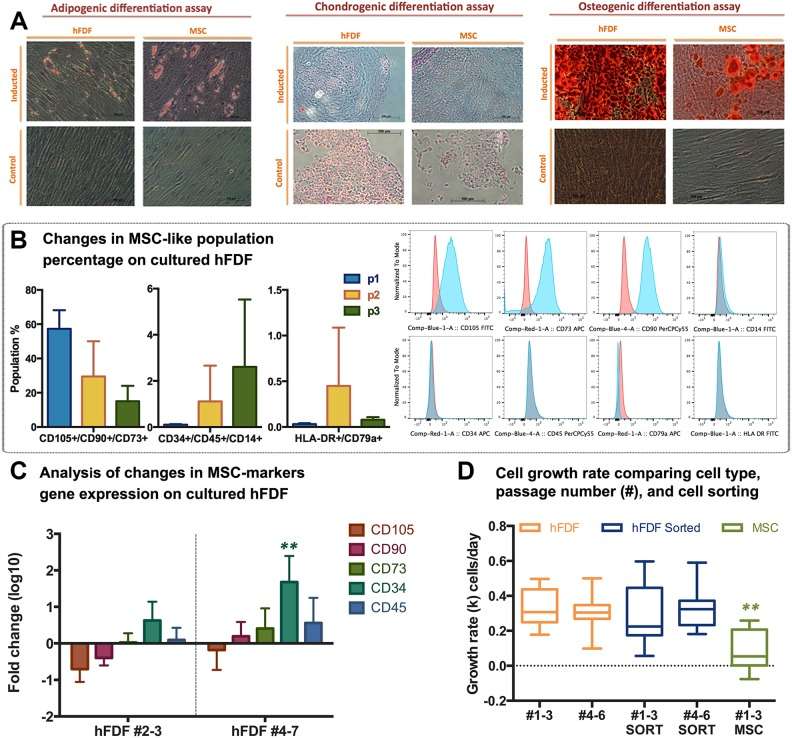 Fig. 1. Detection of mesenchymal stem cell (MSC) population in human fetal dermal-derived fibroblasts (hFDFs) (Esteban-Vives R, Ziembicki J, et al., 2019).
Fig. 1. Detection of mesenchymal stem cell (MSC) population in human fetal dermal-derived fibroblasts (hFDFs) (Esteban-Vives R, Ziembicki J, et al., 2019).
Traction Forces Lower in Fetal Dermal Fibroblasts Than Adult
During adult wound healing, fibroblasts transition into myofibroblasts, generating forces that lead to scar formation. However, fetal wounds, which contain less transforming growth factor-ß1 and are softer, heal without scarring. Thus, Rachel's team explored whether the unique contractile behavior of fetal fibroblasts contributes to scarless repair.
Rachel's team tested whether physiological wound rigidity induces contractile forces in fetal dermal fibroblasts. Their previous work suggests that fetal fibroblasts have altered responses to ECM mechanical cues; therefore, they tested whether mechanosensing by adult and fetal dermal fibroblasts is differentially regulated by ECM rigidity. Fibroblasts were cultured on fibronectin-conjugated soft, hard, and rigid PAAs, corresponding to various wound healing stages, to measure contractile force generation (Fig. 2A). Both cell types showed similar force generation on soft and hard PAAs, but adult dermal fibroblasts exerted more force on rigid PAAs (Fig. 2B-C). This difference persisted after 18 hours (Fig. 2A-C). Using traction force microscopy, they once again found that adult dermal fibroblasts exerted larger traction forces than fetal dermal fibroblasts on rigid PAAs even though their cell sizes were the same (Fig. 2D-F). The results showed that traction forces were higher in adult dermal fibroblasts than in fetal ones after incubation.
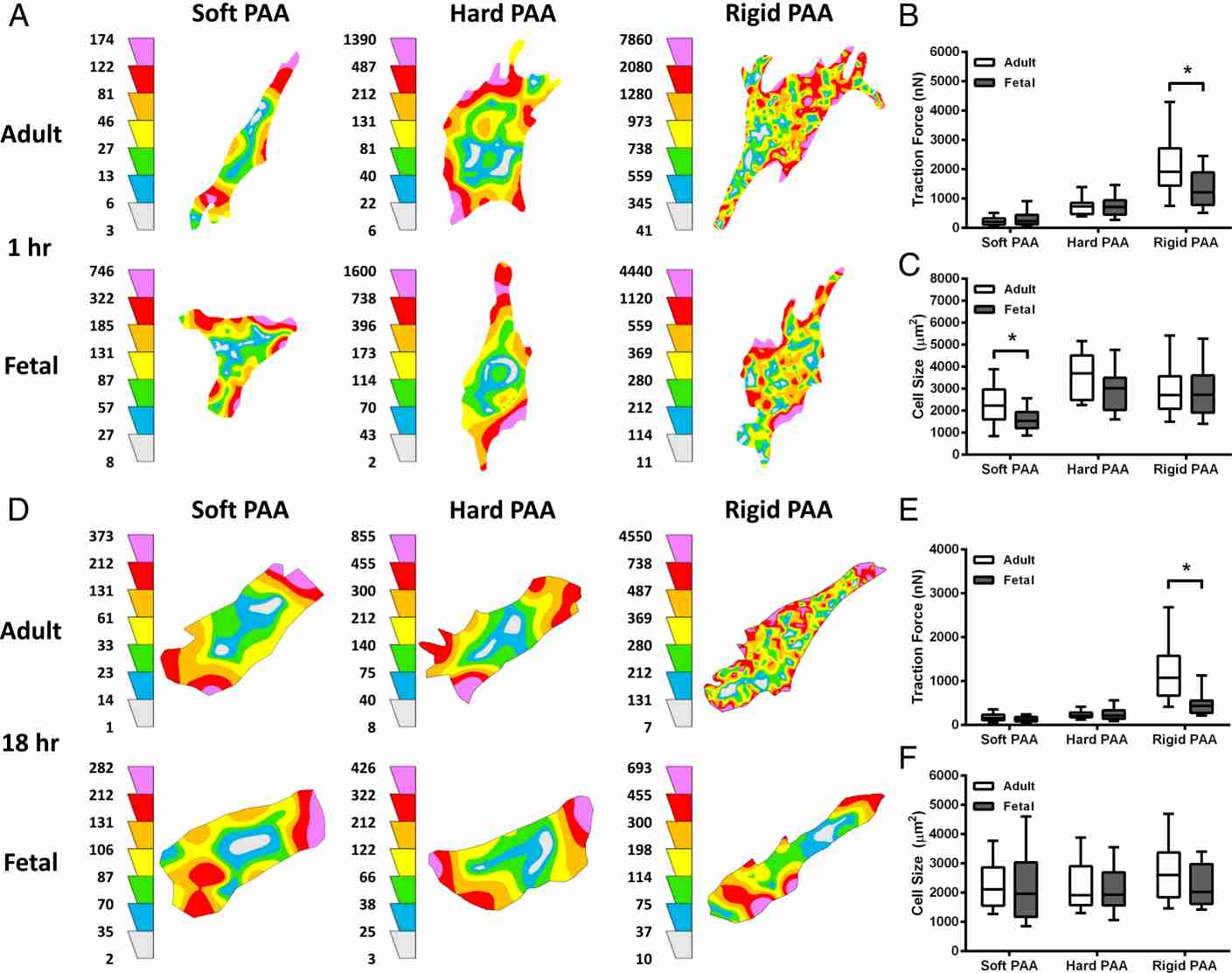 Fig. 2. Fetal dermal fibroblasts exert less contractile force than adult dermal fibroblasts on rigid ECM (Rachel J., Jerrell, BS., et al., 2018).
Fig. 2. Fetal dermal fibroblasts exert less contractile force than adult dermal fibroblasts on rigid ECM (Rachel J., Jerrell, BS., et al., 2018).
Ask a Question
Write your own review