ONLINE INQUIRY
HP2 Heps 10 Human Pooled Primary Hepatocytes
Cat.No.: CSC-C4108X
Species: Human
Source: Liver
Cell Type: Hepatocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
Each donor's hepatocytes have been cryopreserved ONCE immediately after isolation and purification.
Pools of 10 or 20 donors are available. Each donor is represented by a statistically significant number of cells relative to the pool.
There is extensive quality control providing characterization of viability and enzyme activity from both individual donors and the total pool.
Creative Bioarray guarantees performance and quality.
Donor information is available.
Human hepatocytes are the largest parenchymal cells of the human liver: more than 2.5 billion hepatocytes, more than half a million liver lobules make up an adult liver. They maintain a multitude of metabolic, detoxifying and synthetic processes, are engaged in several key physiological processes, and contribute to liver homeostasis. Because of their pharmacological function for detoxification and metabolism, primary hepatocytes are frequently employed as in vitro liver models for drug metabolism and pharmacokinetics. They are the gold standard, and could be used to transplant human hepatocytes and create bioartificial livers.
But single-donor hepatocytes have their drawbacks. Metabolic features of a single-donor hepatocyte may not reflect the general population, as activity and expression levels of drug-metabolizing enzymes differ significantly from one individual to another. This variability can be misleading when it comes to predicting drug responses. The use of pooled donor primary hepatocytes can address this limitation. Pooled hepatocytes better reflect the metabolic capacity of the general population, thereby reducing experimental bias caused by genetic differences among individuals. This approach provides a more comprehensive metabolic profile and drug response pattern. Moreover, because the liver contains multiple types of cells, aggregation of hepatocytes can better replicate the diverse in vivo hepatic environment. Furthermore, when creating bioartificial organs, pooled hepatocytes can also improve the function and plasticity of these organs. In this way, pooling pooled hepatocytes for specific research needs can facilitate better, more accurate research.
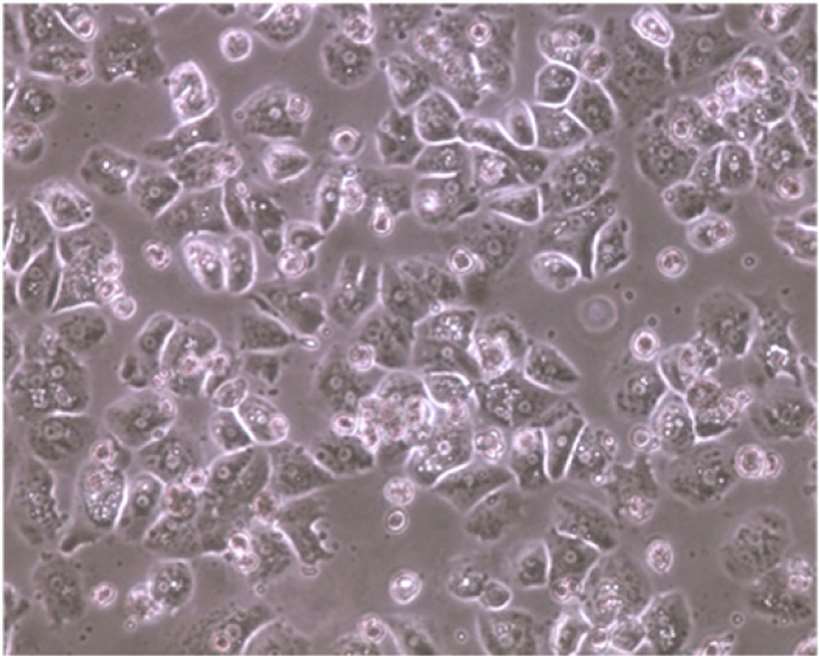 Fig. 1. Morphology of pooled cryopreserved human hepatocytes (Li A. P., 2015).
Fig. 1. Morphology of pooled cryopreserved human hepatocytes (Li A. P., 2015).
Determination of Metabolites in Plasma of Volunteers and Pooled Hepatocytes Cultures
Amiodarone (ANT) is a first-generation antiarrhythmic and anticholinergic medication, which has already seen widespread clinical use. Several recent trials indicate that the drug is also highly effective in stopping atrial fibrillation. But it hasn't entered clinical practice, and there is no information on how it is metabolized in the human body. Therefore, Giebułtowicz J et al. analyzed the metabolic profile of intravenously injected ANT in plasma and human hepatocyte pools by liquid chromatography tandem mass spectrometry.
In the chromatograms of plasma from volunteers, 15 potential ANT metabolites were identified. The majority of the metabolites that were detected in volunteer plasma were also observed in in vitro experiments using pooled human hepatocytes. These metabolites consist of Phase I compounds such as M1, M2, and the metabolites MW295 and MW299, as well as Phase II compounds such as methylated and glucuronidated compounds M6, MW485, and MW487b. Some metabolites are labelled with glucuronic acid (M3, M4, M5, M7, M8, MW351, MW475) or sulfuric acid (MW361). The primary metabolic routes for the ant compound are described in Figure 1, which include cleavage, hydroxylation and conjugation. Some specific metabolites, like compounds B and C, were found under certain conditions but did not meet criteria for significant metabolite selection in in vivo studies. The study showcases the intricate pathways of ANT metabolism involving multiple transformations and conjugations.
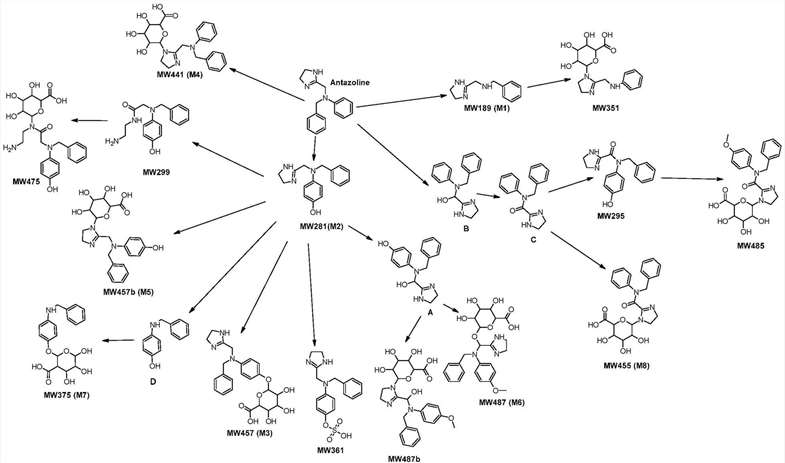 Fig. 1. The main pathways of ANT metabolism and putative structure of Phase I and Phase II metabolites (Giebułtowicz J, Korytowska N, et al., 2020).
Fig. 1. The main pathways of ANT metabolism and putative structure of Phase I and Phase II metabolites (Giebułtowicz J, Korytowska N, et al., 2020).
Gene-Level Analysis of Pooled Human Hepatocytes Exposed to Thioacetamide
The employment of pooled hepatocyte cultures in in vitro experiments to study the metabolism, toxicity, and drug interactions of chemicals can reduce variability. Toxicogenomics aids in understanding how gene expression patterns and subsequent affected pathways characterize physiological responses to drugs and toxins. However, the utility or applicability of pooled human hepatocytes in predicting hepatotoxicity remains unclear. Therefore, the objective of this study is to evaluate the response of pooled human hepatocytes to thioacetamide exposure. Goel H, et al. examined RNA-seq data from five individual lots of pooled primary human hepatocytes, as well as a combined lot, following thioacetamide exposure, and analyzed the homogeneity and variability of these pooled hepatocyte cultures using various toxicogenomic approaches.
Through RNA sequencing data analysis, the researchers identified gene expression responses induced by harmful substance exposure. The number of genes in different lots of pooled hepatocytes varied significantly, ranging from 2,241 to 3,521. Additionally, there was a substantial overlap of genes among the different lots, with 816 common differentially expressed genes, indicating similarity in genes affected by thioacetamide (Fig. 2). Studying these genes can help focus on their potential roles in liver function and damage. Notably, the downregulated genes CYP26B1 and CYP26A1 are responsible for the clearance of retinoic acid in the liver. When thioacetamide was administered to rats, elevated retinoic acid levels and reduced activity of related enzymes were observed. The upregulated genes C2CD4B and C2CD4A primarily participate in the regulation of inflammatory responses. SPP1 and THRSP were also among the top 10 differentially expressed genes, with SPP1 being an important biomarker for fibrosis and FGF21 playing a crucial role in lipid metabolism and reducing hepatocyte damage. Furthermore, several genes like SPP1 and THRSP are associated with liver injury.
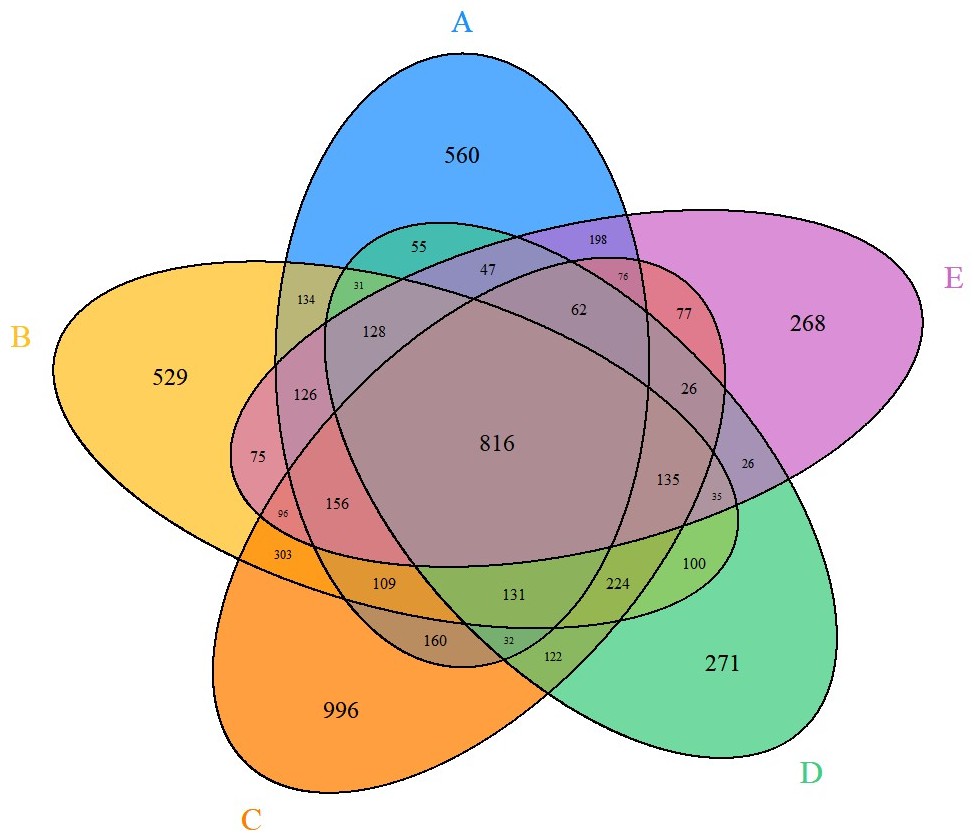 Fig. 2. Venn diagram depicting the common differentially expressed genes across different lots of in vitro pooled human hepatocytes exposed to thioacetamide treatment (Goel H, Printz RL, et al., 2024).
Fig. 2. Venn diagram depicting the common differentially expressed genes across different lots of in vitro pooled human hepatocytes exposed to thioacetamide treatment (Goel H, Printz RL, et al., 2024).
Ask a Question
Write your own review







