ONLINE INQUIRY
HP1 Heps-i Human Primary Hepatocytes-Single Donor
Cat.No.: CSC-C4112X
Species: Human
Source: Liver
Cell Type: Hepatocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
Suitable for induction assays or metabolic characterization assays in plated or suspension cultures. Plated cultures are excellent for long-term intrinsic clearance assays.
Hepatocytes are cryopreserved ONCE immediately after isolation and purification.
Characterization of viability and enzyme activity, including fold-induction of CYP1A2, CYP2B6 and CYP3A4 for HP1 Heps-i.
Creative Bioarray guarantees performance and quality.
Donor demographics and medical information are available.
Human primary hepatocytes are highly differentiated cells isolated from adult liver tissue. Under specific in vitro culture conditions, these cells can retain their biological functions, including roles in cellular metabolism, detoxification, and protein synthesis. They serve as pivotal tools for studying liver physiology and pathological mechanisms. Creative Bioarray's HP1 Heps-i human primary hepatocytes-single donor are induced hepatocytes derived from primary human liver cells, achieved by adding exogenous small molecules or growth factors. These external factors promote cell proliferation and extend the passaging capacity of the cells, making them suitable for long-term culture conditions required in prolonged drug induction studies or metabolic research.
This capability for extended culture provides several advantages. Firstly, these cells typically exhibit stable growth and function, offering consistent experimental conditions that reduce variability and enhance data reproducibility. Secondly, they enable long-term experimental observations, which are crucial for studying chronic toxicity, pharmacokinetics (PK), and long-term drug effects. In addition, these cells can be used to establish in vitro disease models, aiding in the investigation of pathological mechanisms and drug screening. Finally, their potential for large-scale expansion ensures that they can meet the demands of extensive experimental setups. In summary, the prolonged expansion and sustained functionality of human primary hepatocytes in vitro provide valuable tools and methodologies for research in regenerative medicine, drug screening, and cellular therapy.
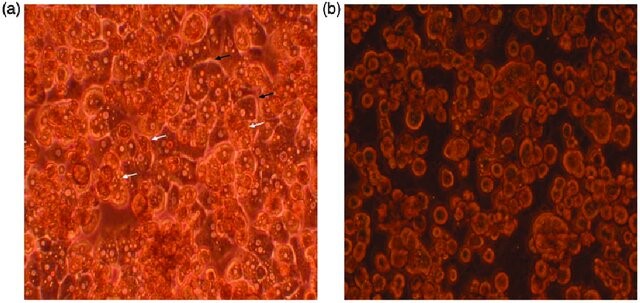 Fig. 1. Primary human hepatocytes on day 3 of in vitro culture (Baloch, K., Chen, L., et al., 2017).
Fig. 1. Primary human hepatocytes on day 3 of in vitro culture (Baloch, K., Chen, L., et al., 2017).
DEX Reduces RDV-Induced Cytotoxicity in Human Primary Hepatocytes
Remdesivir (RDV) is an FDA-approved antiviral drug for the treatment of COVID-19 patients. Dexamethasone (DEX) is a corticosteroid commonly co-administered with RDV. The drug interactions and hepatotoxicity between RDV and DEX remain unknown. Liu's team elucidates the hepatotoxicity of RDV and its interaction with DEX. Findings suggest that the combination of DEX and RDV could potentially reduce the likelihood of RDV-induced liver injury in hospitalized COVID-19 patients.
They studied RDV's impact on cell viability using human primary hepatocytes (HPH) in 2D and 3D cultures. RDV reduced cell viability dose-dependently, with CC50 values of 7.5 to 22.66 μM in 2D and 4.24 to 7.96 μM in 3D (Fig. 1A and B). Testing whether dexamethasone (DEX) affects RDV-induced hepatotoxicity, they co-treated hepatocytes with DEX and RDV. DEX at 1 μM maximized protection in both formats, while 5 μM increased caspase-3 cleavage (Fig. 1C). Thus, 1 μM DEX was used in further studies, showing reduced dead/live cell ratios with RDV plus DEX (Fig. 1D). Co-treatment with 1 μM DEX shifted RDV's CC50 values to higher levels in 2D, 3D, and HepG2 cells (Fig. 1E-G). Optimal DEX protection was at RDV exposures of 5-20 μM but diminished above 40 μM. Overall, RDV is hepatotoxic, with 3D models more sensitive than 2D, and DEX mitigates RDV-induced toxicity.
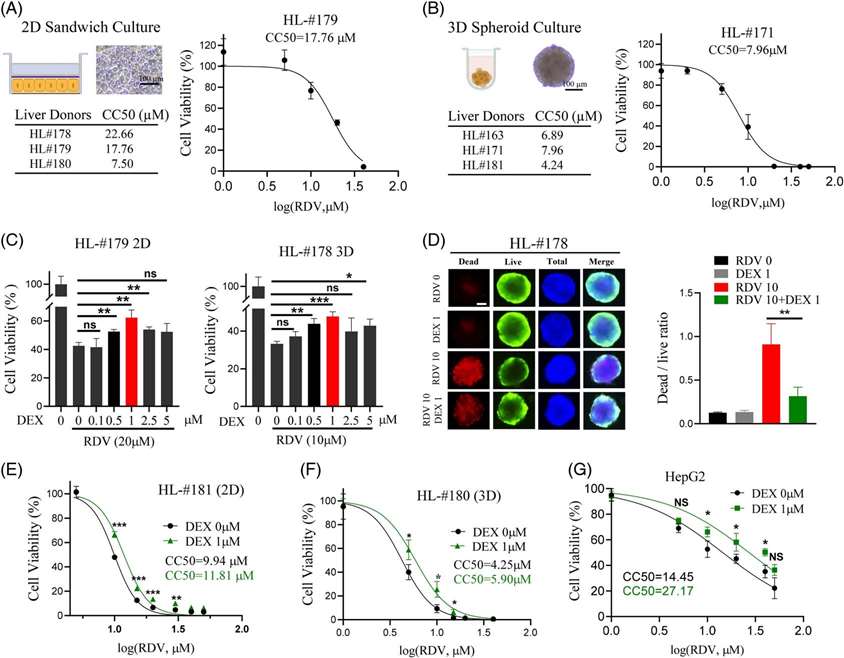 Fig. 1. Dexamethasone reduces remdesivir-induced cell death in human hepatocytes and HepG2 cells (Liu K, Stern S, et al., 2023).
Fig. 1. Dexamethasone reduces remdesivir-induced cell death in human hepatocytes and HepG2 cells (Liu K, Stern S, et al., 2023).
LncRNA TUG1 was Involved in Sepsis-Induced Liver Injury and Mitochondrial Dysfunction Improvement Triggered by Rg3
Previous studies have demonstrated that mitochondrial damage correlates with the clinical severity of sepsis, and inhibiting mitophagy can improve patient outcomes. Ginsenoside Rg3, a steroidal saponin extracted from ginseng, possesses anticancer and anti-inflammatory properties. Evidence suggests that Rg3 can enhance autophagy, thereby preventing sepsis-induced cellular and organ damage as well as mitochondrial dysfunction; however, the specific mechanisms related to hepatic injury remain to be elucidated. Long non-coding RNA (lncRNA) taurine up-regulated gene 1 (TUG1) is considered a regulatory factor in sepsis. Wu's team aims to investigate the relationship between TUG1 and Rg3.
Wu's team first established a standard mouse model of sepsis and demonstrated the association between TUG1 and the occurrence of sepsis through this model (Fig. 2A-D). Then investigated the effects of Rg3 on LPS-induced human primary hepatocytes. CCK-8 assay showed that pretreatment with Rg3 increased the viability of LPS-induced human primary hepatocytes (Fig. 2E). Furthermore, Rg3 also ameliorated LPS-induced downregulation of the protein levels of complex I, complex II, and OPA1 in hepatocytes (Fig. 2F-G). LPS-induced downregulation of TUG1 expression in LPS-induced hepatocytes was largely recovered by Rg3 pretreatment (Fig. 2H).
Then, they investigated TUG1's role in Rg3-improved mitochondrial dysfunction in LPS-treated hepatocytes. TUG1 expression dropped significantly in LPS-treated hepatocytes with sh-TUG1 transfection (Fig. 3A). Rg3 boosted LC3 II/LC3 I and Beclin-1 levels, but TUG1 knockdown reduced this effect, while p62 levels stayed constant (Fig. 3B-C). Rg3 pretreatment lessened LPS-induced mitochondrial ROS production, but this was reversed by TUG1 knockdown. Rg3 also lowered LPS-induced green fluorescence, indicating low MTP levels, which was counteracted by silencing TUG1 (Fig. 3E and F). Furthermore, Rg3-induced downregulation of OPA1, complex I, and complex II was impaired by TUG1 knockdown (Fig. 3G and H). Western blot confirmed Rg3 raised PGC1-α, NRF-1, and Tfam levels, which TUG1 knockdown attenuated (Fig. 3I and J). These results suggest Rg3 enhances autophagy to alleviate mitochondrial dysfunction in LPS-treated hepatocytes by upregulating TUG1.
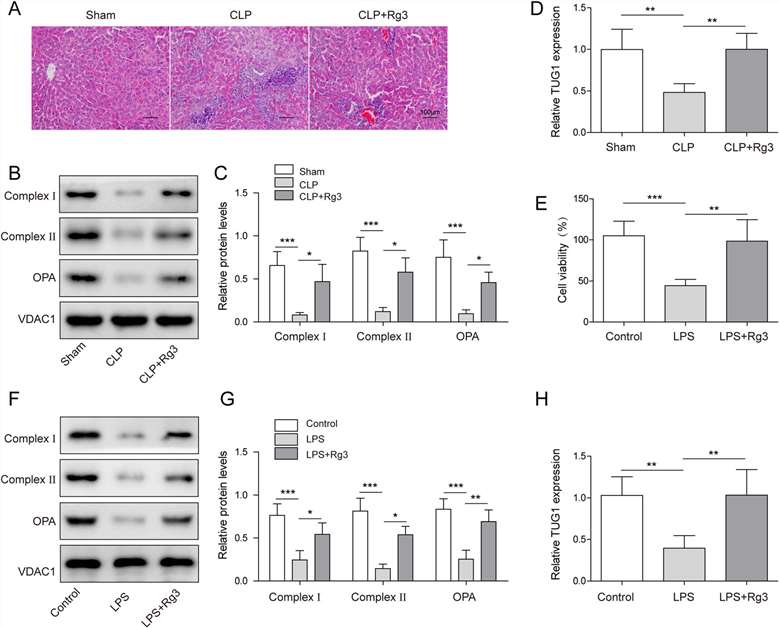 Fig. 2. LncRNA TUG1 was involved in sepsis-induced liver injury and mitochondrial dysfunction improvement triggered by Rg3 (Wu P, Yu X, et al., 2021).
Fig. 2. LncRNA TUG1 was involved in sepsis-induced liver injury and mitochondrial dysfunction improvement triggered by Rg3 (Wu P, Yu X, et al., 2021).
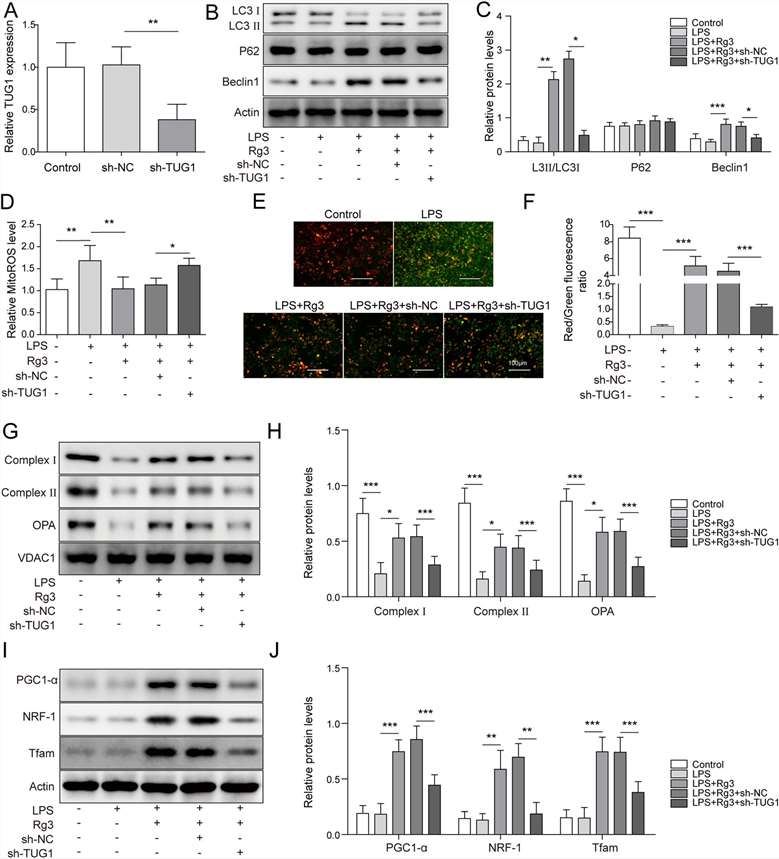 Fig. 3. TUG1 knockdown almost reversed Rg3-induced mitochondrial dysfunction improvement in LPS-treated hepatocytes (Wu P, Yu X, et al., 2021).
Fig. 3. TUG1 knockdown almost reversed Rg3-induced mitochondrial dysfunction improvement in LPS-treated hepatocytes (Wu P, Yu X, et al., 2021).
Ask a Question
Write your own review







