ONLINE INQUIRY
Fetal Liver Cells Mononuclear Cells 13-24 Weeks Gestation
Cat.No.: CSC-C4499X
Species: Human
Source: Liver
Cell Type: Mononuclear Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Fetal liver mononuclear cells (FLMNCs) are essential for the formation of the immune system and embryonic hematopoiesis. These cells typically are round or oval in shape. The mononuclear precursor cells have larger nuclei and fewer cytoplasm; the juvenile mononuclear cells have more cytoplasm, a pale blue; and the adult mononuclear cells have plenty of cytoplasm and lysosomes. These cell's distinctive morphological signatures are readily seen through the microscope.
During the 13-24 weeks of fetal liver development, hematopoietic roles are becoming increasingly streamlined and stable in multiple organs as the fetal grows. In this phase, the bone marrow gradually replaces the heart as the center of hematopoiesis, and the liver begins to act as a co-organizer in hematopoiesis and immunity. Now the liver's mononuclear cells become larger and diverse in their composition and function, both mononuclear precursor cells and mature functional mononuclear. These cells can then develop into macrophages with greater phagocytic capabilities and more elaborate immune responses. They increasingly engage in intrahepatic immune regulation, defense against pathogens, local tissue repair and organ homeostasis.
FLMNCs are broadly used in scientific research, especially hematopoiesis. They are also helping to uncover the function of mononuclear cells in the hepatic hematopoietic microenvironment, contributing to our understanding of hematopoietic stem cell growth and function. And those cell lines are crucial to understanding fetal immune system development, offering fundamental insights into the immune system maturation.
GC-macrophages Share Characteristics with CD163+ Macrophages Found in Human Bone Marrow and Fetal Liver
Erythropoiesis in human bone marrow (BM) and fetal liver (FL) occurs within erythroblastic islands, comprising a central macrophage surrounded by erythroid cells at different maturation stages. Macrophages are crucial in this process, but existing models are limited to BM harvesting or complex genetic modifications. Previous studies showed that cultured monocytes can differentiate into macrophages that support erythropoiesis and hematopoietic stem and progenitor cells (HSPC) survival, resembling BM and FL macrophages. Heideveld et al. aimed to establish a convenient in vitro model to investigate erythroid-macrophage interactions by differentiating monocytes into anti-inflammatory M2 macrophages (GC-macrophages) using glucocorticoids, to mimic erythroblastic islands in vitro.
They analyzed whether macrophages in primary erythropoietic organs (human fetal liver (FL) and bone marrow (BM)) share phenotypic characteristics with GC-macrophages. During weeks 15-22, the FL is heavily involved in erythropoiesis. FL exhibits increased CD71+CD235- pro-erythroblasts (Fig. 1A and B). To prevent free immunogenic pyrenocytes and support erythroid cells in embryos, FL contains substantial erythroblastic islands and macrophages, showing a 6.5-fold increase in CD163+ macrophages compared to BM (Fig. 1C and D). Both FL and BM macrophages express high CD163 and CD14, with CD163+ FL macrophages showing higher CD16 expression and CD163+ BM macrophages higher CXCR4. GC-macrophages mimic both BM and FL macrophages, showing similarities to BM macrophages (CD16 and CXCR4) and FL macrophages (CD206). Unstimulated cells lack VCAM1 and display low CD206, CD163, CD14, and CD16 (Fig. 1E and F). Both BM and FL CD163+ macrophages bind erythroid cells, indicating CD163 marks erythroid-supporting macrophages. FL macrophages interact more with CD71+CD235a+ cells. The phenotypic similarity and erythroid cluster formation of BM, FL, and GC-macrophages suggest GC-macrophages share key characteristics with in vivo erythroid-supporting macrophages and could serve as an in vitro model for studying macrophage support in erythropoiesis.
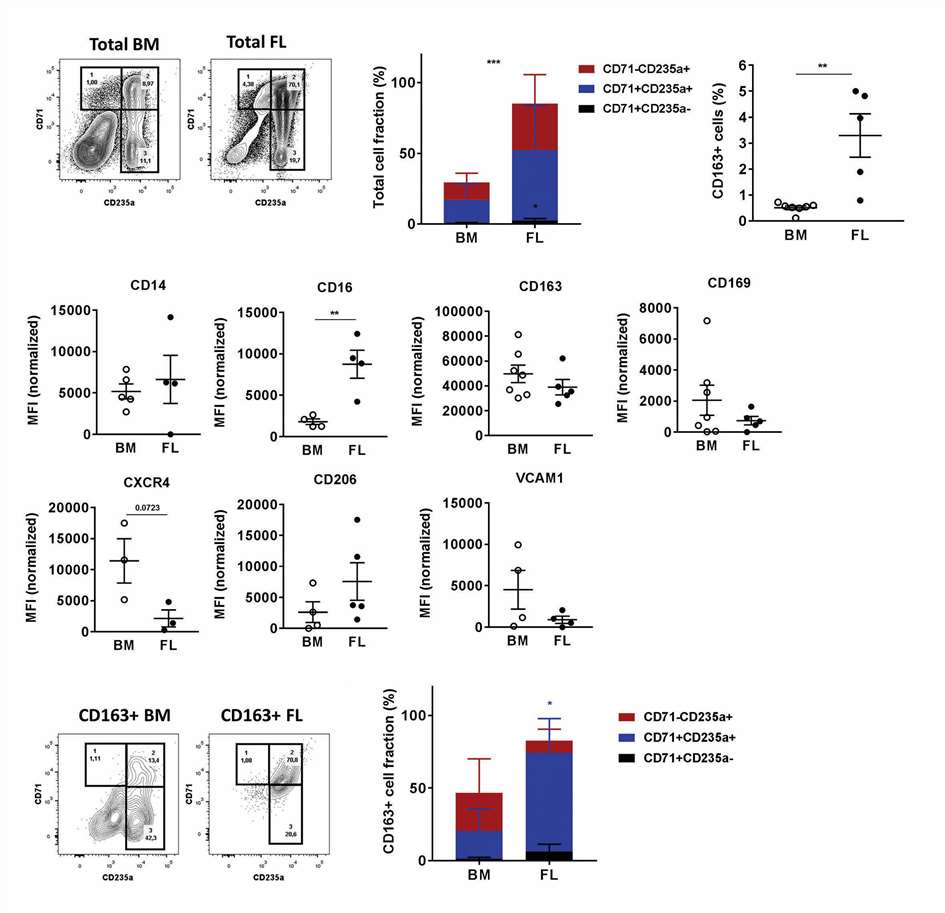 Fig. 1. CD163+ macrophage populations in human BM and FL (Heideveld E, Hampton-O'Neil LA, et al., 2018).
Fig. 1. CD163+ macrophage populations in human BM and FL (Heideveld E, Hampton-O'Neil LA, et al., 2018).
HMGA2 is Preferentially Expressed in the Most Immature Fractions of Human
hematopoietic stem and progenitor cells (HSPCs)
Hematopoietic stem cells (HSCs) are crucial for generating differentiated blood cells throughout life. Regulation of their fate is controlled by various DNA-binding factors. High-mobility group AT hook 2 (HMGA2) is a nonhistone chromosomal-binding protein that has shown significant roles in regulating stem and progenitor cells, influencing gene expression, chromatin conformation, and recruiting factors for transcription. Kumar et al. aimed to elucidate the role of HMGA2 in the regulation of human HSCs by investigating its effects on proliferation and differentiation using both gain-of-function and loss-of-function approaches.
Mononuclear cells were isolated from neonatal cord blood (CB) and adult bone marrow (BM) using density gradient separation, followed by CD34+ cell isolation with anti-CD34 magnetic beads along with human fetal liver mononuclear cells (16 weeks). CB CD34 cells were then stained with CD34-fluorescein isothiocyanate, CD38-phycoerythrin, CD90-allophycocyanin, CD45RA-V450 for HSCs sorting. Results showed higher HMGA2 expression in the most primitive hematopoietic fractions (HSCs and multipotent progenitors) than in more committed progenitors across all three stages (Fig. 2A-C). Additionally, HSCs from CB and fetal liver had significantly higher HMGA2 expression compared to adult BM HSCs (Fig. 2D), consistent with previously observed fetal stage-specific expression in murine hematopoiesis. Since HMGA2 is posttranscriptionally regulated by Let-7 microRNAs, they validated the expression at the protein level, confirming robust expression in CB-derived HSCs and MPPs but almost undetectable in more mature cells (Fig. 2E). This protein analysis further showed that HSCs had substantially higher HMGA2 expression compared with MPPs that lack self-renewal potential, indicating HMGA2's role in regulating HSC function.
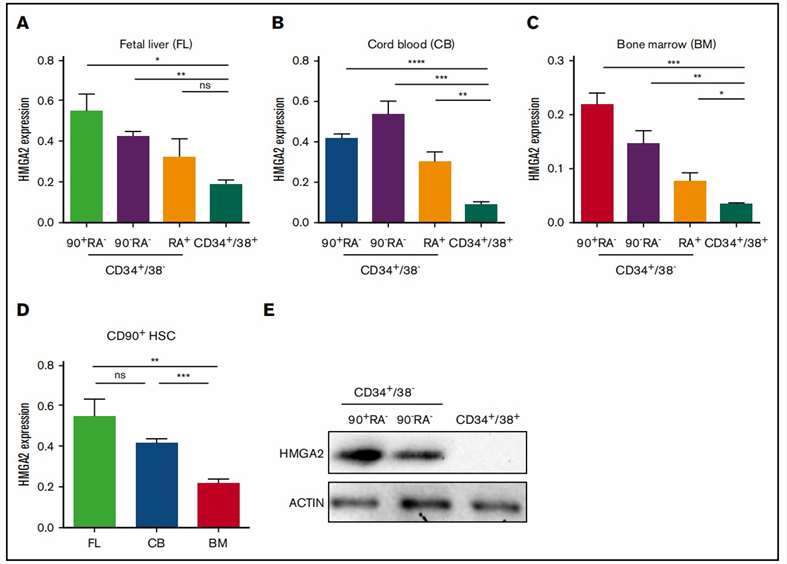 Fig. 2. Preferential expression of HMGA2 within the primitive compartment of human hematopoietic cells (Kumar P, Beck D, et al., 2019).
Fig. 2. Preferential expression of HMGA2 within the primitive compartment of human hematopoietic cells (Kumar P, Beck D, et al., 2019).
Ask a Question
Write your own review