ONLINE INQUIRY
Porcine Cardiac Fibroblasts
Cat.No.: CSC-C4886L
Species: Pig
Source: Heart
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
PCFs originate from pig heart tissue with their primary source being fibrous areas including the epicardium, endocardium, and myocardial interstitium. The main component cells among these cardiac fibroblasts are epicardial fibroblasts. Porcine cardiac fibroblasts provide essential support to heart structure and repair while also contributing to heart disease progression. Mechanical stability is preserved by PCFs through their production and release of extracellular matrix components including collagen. During cardiovascular conditions such as myocardial infarction and hypertension PCFs actively drive cardiac remodeling and fibrosis through secretion of pro-inflammatory cytokines and transforming growth factor beta (TGF-β). Scientists acknowledge PCFs as essential tools for investigating cardiovascular disease mechanisms because they enable research into cardiac remodeling processes as well as fibrosis and post-infarction repair. Research reveals that targeted small molecules or transcription factors successfully transform PCFs into cardiac muscle cell analogs thus providing innovative methods for heart regenerative treatments.
CircRNAs in Doxorubicin- and Myocet-Induced Cardiotoxicity in pCPCs and pCFs
Anthracycline-induced cardiotoxicity remains a major clinical challenge despite liposomal DOX (MYO) reducing myocardial drug accumulation. CircRNAs, known to sponge miRNAs and regulate pathways in cancer, are underexplored in chemotherapy-related cardiac damage.
Mester-Tonczar et al. employed a translational pig model treated with DOX or MYO, combining bulk mRNA-seq and CIRIquant for circRNA identification. Then, two candidate circRNAs (circ-MT:3033|3289 and circ-MT:3070|3478) were validated in porcine cardiac progenitor cells (pCPCs) and porcine cardiac fibroblasts (pCFs). In pCPCs, circ-MT:3033|3289 expression was significantly downregulated in MYO-treated groups versus controls and DOX-treated cells (Fig. 1A). Conversely, in pCFs, MYO and DOX treatments upregulated circ-MT:3033|3289 compared to controls (Fig. 1B). No significant changes were observed for circ-MT:3070|3478 in either cell type (Fig. 1C and D). These results suggest MYO exerts a stronger regulatory effect on circ-MT:3033|3289 than DOX. Sanger sequencing confirmed mitochondrial circRNA backsplice junctions (Fig. 2A and B).
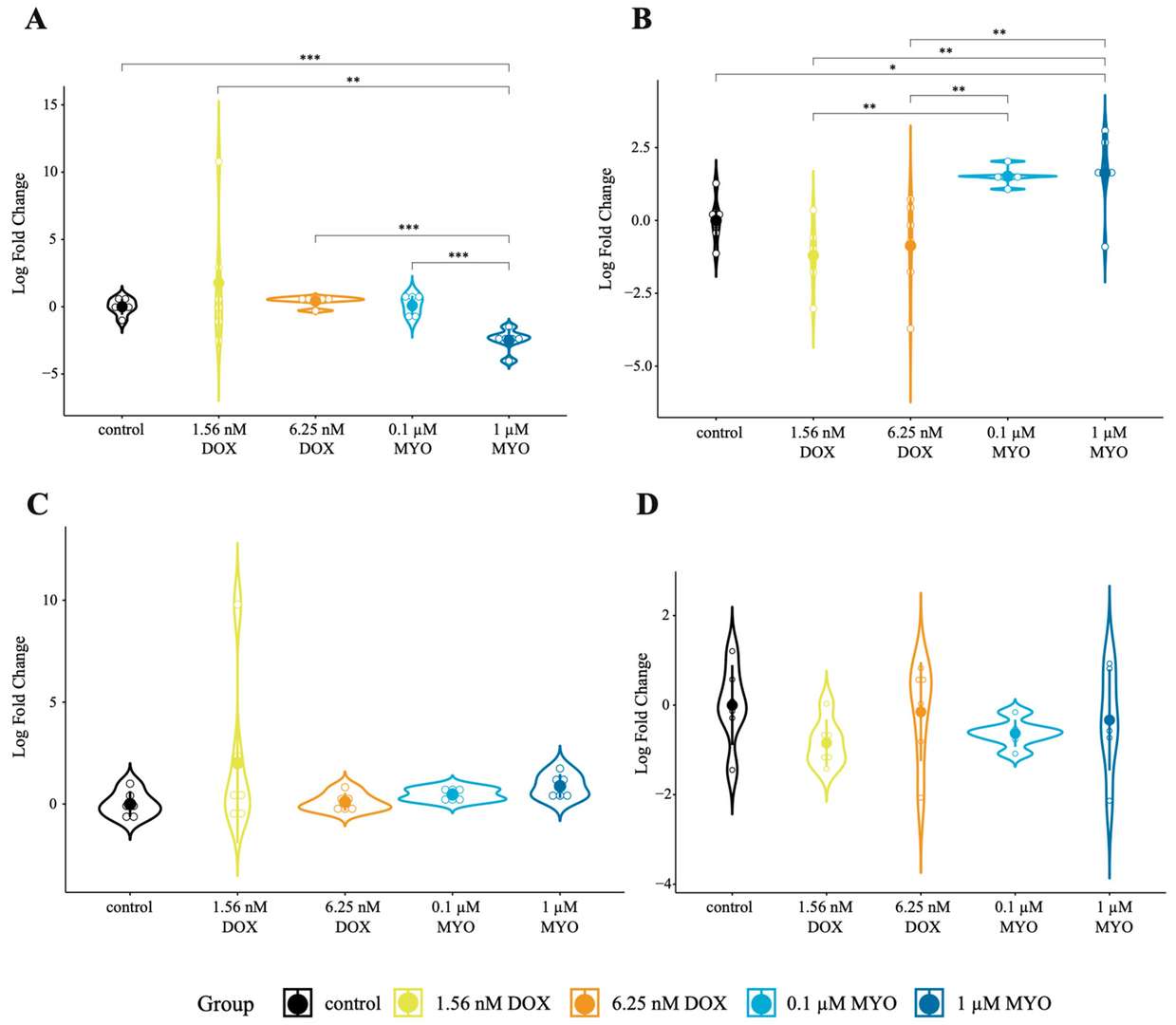 Fig. 1. In vitro expression of mt-circRNAs in DOX- and MYO-treated cells (Mester-Tonczar J, Einzinger P, et al.,2023).
Fig. 1. In vitro expression of mt-circRNAs in DOX- and MYO-treated cells (Mester-Tonczar J, Einzinger P, et al.,2023).
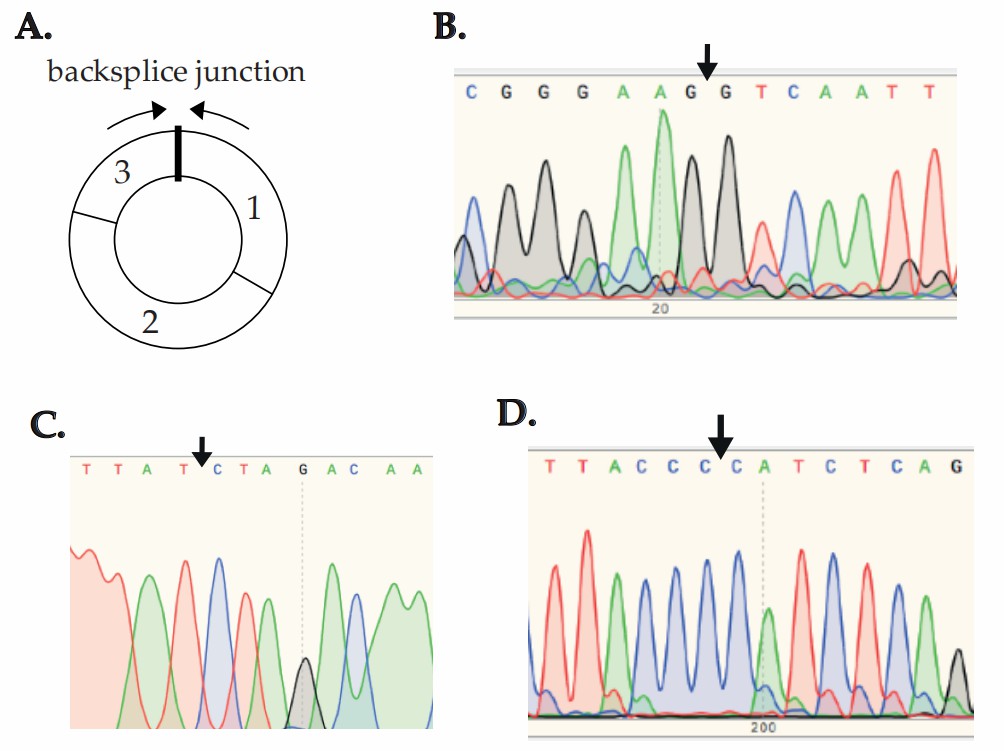 Fig. 2. The presence of backsplice junctions (BSJ) of circRNA in cell culture experiments confirmed using Sanger sequencing (Mester-Tonczar J, Einzinger P, et al.,2023).
Fig. 2. The presence of backsplice junctions (BSJ) of circRNA in cell culture experiments confirmed using Sanger sequencing (Mester-Tonczar J, Einzinger P, et al.,2023).
Treatment With CHIR + FGF1 Enhances Cell Cycle Progression of Vascular Cells and Fibroblasts In Vitro
AMI mortality correlates with infarct size, as cardiomyocyte regeneration is minimal, making myocyte salvage crucial. Fan's team investigated using nanoparticles (NPs) for sustained chemical release, specifically FGF1 and CHIR99021, to promote myocardial protection and infarct size reduction.
To investigate chemical-mediated cardioprotection mechanisms, neonatal cardiomyocytes (NCMs) and vascular cells (ECs, SMCs) were treated with CHIR-FGF1-NPs. Treatment with CHIR, FGF1, or both did not affect BrdU incorporation in NCMs compared to controls. For HUVECs, CHIR + FGF1 significantly increased cell cycle markers (Ki-67, PH3, BrdU, Aurora B) compared to single treatments or controls. Similarly, HVSMCs treated with CHIR + FGF1 showed greater marker expression than single treatments. To determine the effect of CHIR and FGF1 on the cell cycle progression of fibroblasts, the porcine cardiac fibroblasts were isolated, cultured, cell cycle synchronized, and treated with CHIR + FGF1 for 24 hours and assessed again for various cell cycle–associated markers, including BrdU (S phase marker) and PH3 (M phase marker). The incorporation of BrdU and the expression pattern of PH3 are shown in Figure 3, A and B. Quantification of the percentage of vimentin+ (red, vimentin) fibroblasts demonstrated that the expression levels of both markers were significantly higher in the CHIR + FGF1–treated group compared with control groups (Fig. 3A and B).
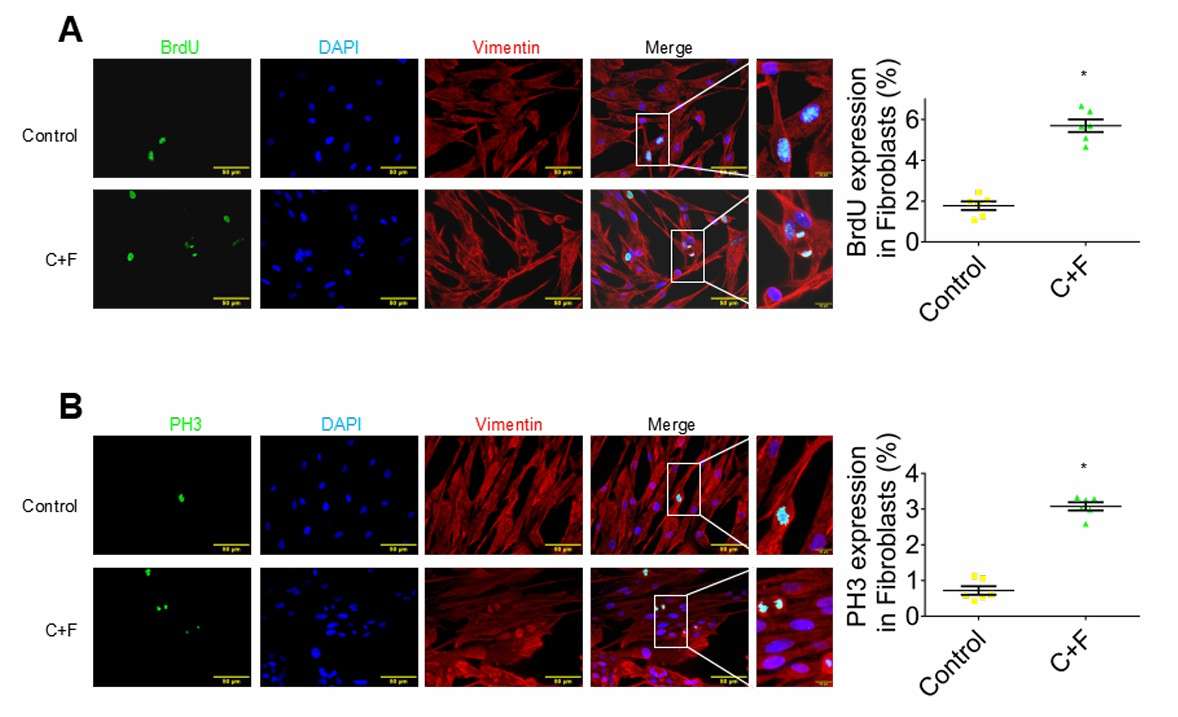 Fig. 3. Assessment of cell cycle in cardiac fibroblasts in vitro (Fan C, Oduk Y, et al., 2020).
Fig. 3. Assessment of cell cycle in cardiac fibroblasts in vitro (Fan C, Oduk Y, et al., 2020).
The culture medium is different for different cells.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Authentic
Creative Bioarray's cell culture products are authentic. The process was very easy to understand and it worked flawlessly.
12 Aug 2023
Ease of use
After sales services
Value for money
Write your own review

