ONLINE INQUIRY

Human Liver Epithelial Cells
Cat.No.: CSC-C4875L
Species: Human
Source: Liver
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Human liver epithelial cells (HLECs) include the epithelial cells in the liver, primarily hepatocytes and cholangiocytes. They are the cells involved in numerous physiological and pathological functions of the liver. Hepatocytes also do a variety of metabolic, detoxifying and synthetic jobs. They are necessary for carbohydrate, fat and protein synthesis and also for excretion of endogenous and exogenous poisons. Additionally, hepatocytes produce and release many plasma proteins, and store glycogen, fats, vitamins and minerals. Cholangiocytes are epithelial cells that are located in the biliary tract system and there are two types, small and large cholangiocytes. They are mostly important for maintaining the integrity of the bile ducts, which includes the bile production and secretion, secretion of cytokines, immune factors, proteins and enzymes, and physical walls within the liver and between liver and biliary system. Also, cholangiocytes are actually regenerative: important for repairing damaged liver and bile ducts.
HLECs have significant potential in clinical settings and scientific areas. In drug design, for example, they are central for investigating drug metabolism and toxicity. For liver disease treatment, HLECs could be used to repair and regenerate liver cells via stem cell transplantation. Furthermore, HLECs are essential for studying the mechanisms and therapeutics of liver disease.
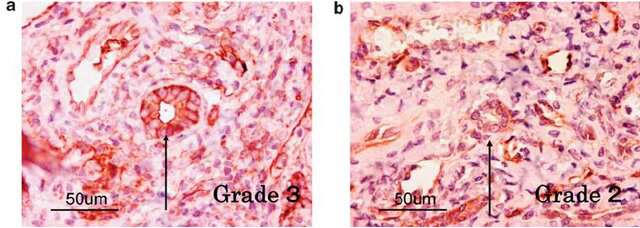 Fig. 1. Human cholangiocytes immunostained with anti-ANXA2 (Kido, O., Fukushima, K., et al., 2009)
Fig. 1. Human cholangiocytes immunostained with anti-ANXA2 (Kido, O., Fukushima, K., et al., 2009)
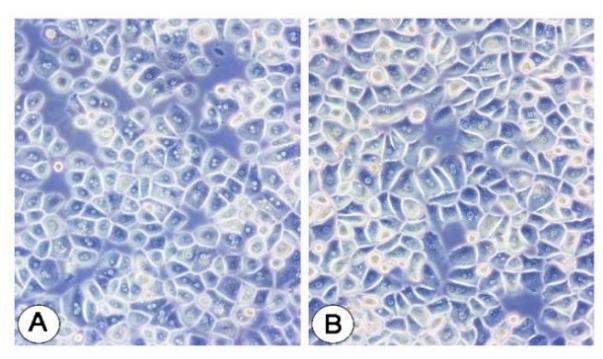 Fig. 2. Human hepatocyte cells (Jeschke, M. G., Klein, D., et al., 2008).
Fig. 2. Human hepatocyte cells (Jeschke, M. G., Klein, D., et al., 2008).
A Decrease in Cell Viability by Exposure to MIT
Methylisothiazolinone (MIT) is widely used in various fields due to its low toxicity and ability to inhibit microbial proliferation. However, increasing evidence suggests that MIT may lead to lung diseases and has been banned from use in humidifier disinfectants, with its toxicity mechanisms remaining unclear. Given that MIT is often used with methylchloroisothiazolinone (MCIT) under the trade name of Kathons, and that intravenously injected Kathons first distributes in the blood and then accumulates in the liver, Park et al. sought to explore the toxicity mechanisms of MIT in human liver epithelial cells. They found that MIT might induce multiple pathways of cell death and inflammatory responses through DNA damage caused by nuclear membrane rupture.
To set cell number and dose-range, Park et al. compared the toxicity of MIT in BEAS-2B (bronchial epithelium), HACAT (keratinocytes) and Chang (liver epithelium) cells which are considered as main target organs of toxicants. They found that the cytotoxic levels depend on the cell number (Fig. 1) and rapidly decrease between 15.625 and 31.25 μg/mL. Based on these results, they chose the highest concentration of 20 μg/mL and the cell number of 2 × 104 cells/mL for viability testing of Chang cells. At exposure of 24 h, the viability of cells exposed with 2.5, 5, 10, and 20 μg/mL of dose was 94.8± 10.7%, 85.2± 16.8%, 84.3±9.2%, and 52.7± 5.5% of control (100.0 ± 8.7%), respectively (Fig. 2).
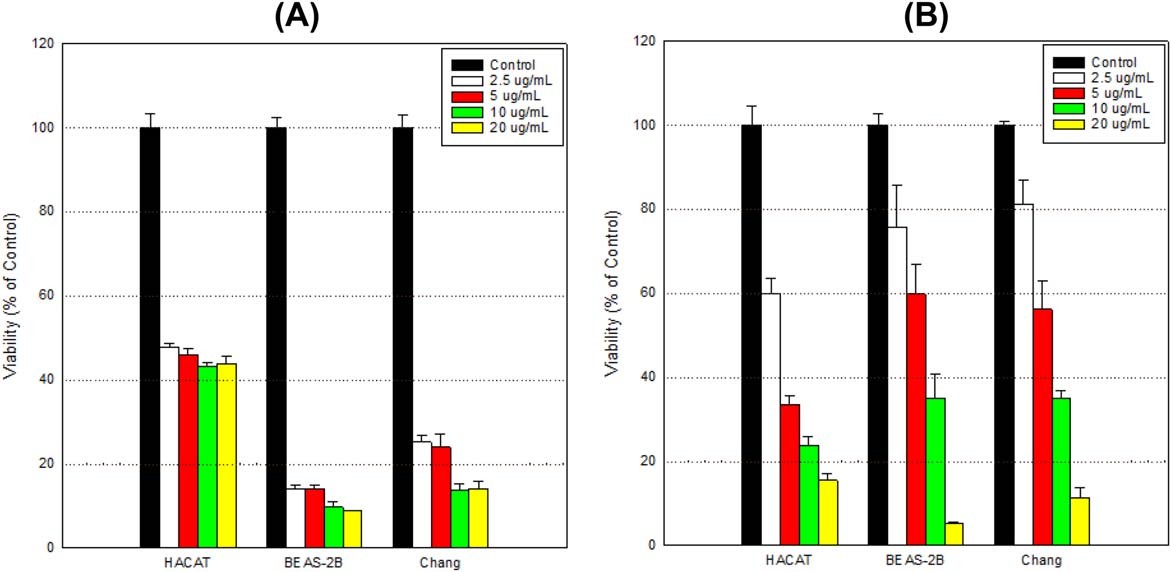 Fig. 1. Comparison of cell viability among cell types by exposure to MIT (Park, E. J., Kim, S., et al., 2018).
Fig. 1. Comparison of cell viability among cell types by exposure to MIT (Park, E. J., Kim, S., et al., 2018).
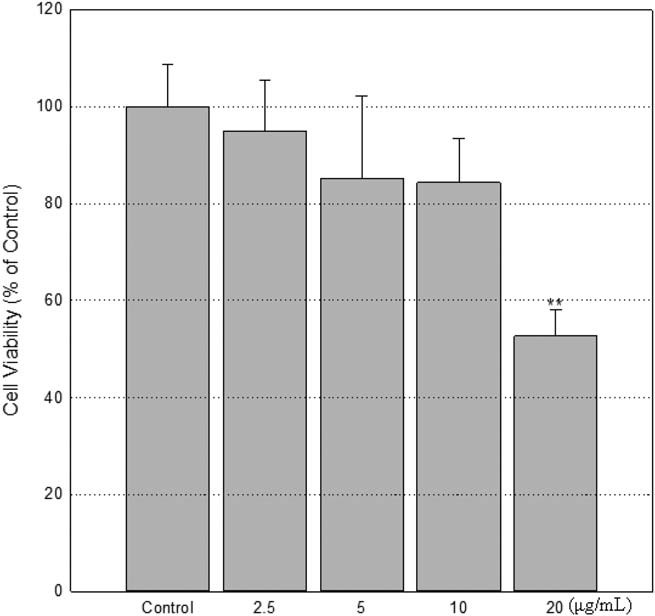 Fig. 2. A decrease of cell viability by exposure to MIT (Park, E. J., Kim, S., et al., 2018).
Fig. 2. A decrease of cell viability by exposure to MIT (Park, E. J., Kim, S., et al., 2018).
Quantification of Liver Epithelial Cell IFN-λ Production in Response to Lipoplex Treatment
The immune system has always been one of the most challenging barriers to overcome in nanoparticle-mediated delivery processes. Type III interferons (IFN-λ1: IL-29, IFN-λ2/3: IL-28A/B) are a family of antiviral cytokines responsible for regulating the functions of epithelial and endothelial cell barriers, supplementing type I interferon responses. Some studies have found that the immune response of IFN-λ is relatively unique compared to existing responses and may not adversely affect the pharmacokinetics and efficacy of nanomedicines. However, there are no studies on the interaction between IFN-λ and nanomedicine delivery. Therefore, this study describes the impact of type III interferons (IFN-λ) on the response of lipid nanoparticles complexed with nucleic acids (lipoplexes), suggesting that IFN-λ pretreatment can enhance the efficacy of chemotherapeutic nanomedicines.
The authors first tested whether human microvascular endothelial cells and/or hepatic epithelial cells respond to IFN-λ by the expression of interferon lambda receptor 1 (IFNLR1). The results showed that the expression levels of IFNLR1 in human hepatic epithelial cells significantly increased after IFN-λ1 or IFN-λ2/3 treatment. This indicates that human hepatic epithelial cells respond to IFN-λ. Meanwhile, the authors also tested whether these cells could produce IFN-λ under liposome treatment. Accordingly, human primary liver epithelial cells were treated with varying amounts of lipoplexes for 24 h and cell media was then collected for determination of IFN-A levels by ELISA. Media from liver epithelial cells treated with 1, 0.5, or 0.25 μg/mL lipoplexes showed an average IFN-λ level of 49.57, 33.52, and 22.42 pg/mL 24 h after treatment, respectively (Fig. 3). However, this latter value is below the lowest concentration that can be reliably quantified by the ELISA (LLoQ = 31.30 pg/mL).
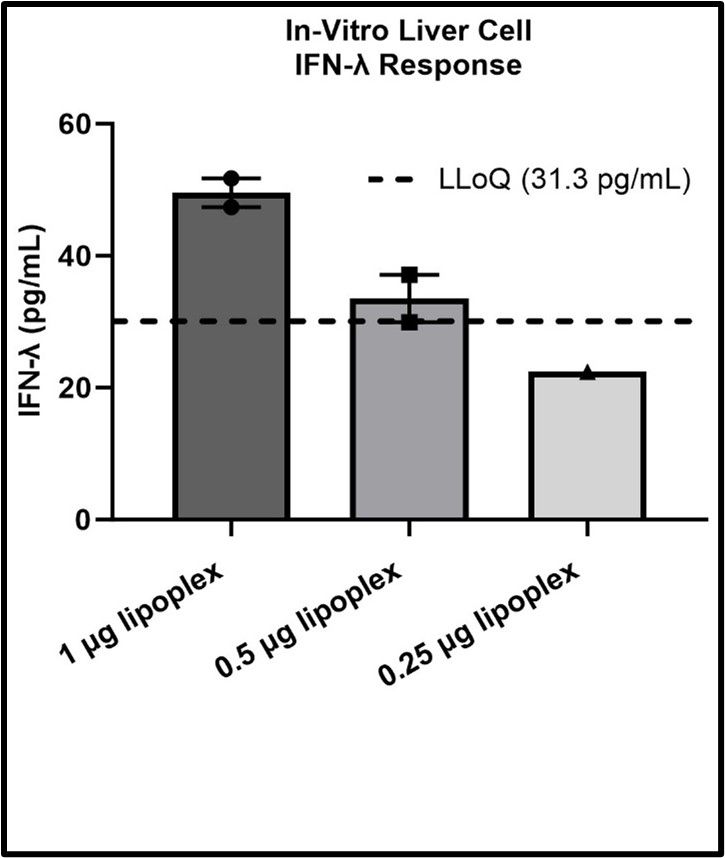 Fig. 3. IFN-λ produced by liver epithelial cells in response to lipoplex exposure (Tilden, S. G., Ricco, M. H., et al., 2024).
Fig. 3. IFN-λ produced by liver epithelial cells in response to lipoplex exposure (Tilden, S. G., Ricco, M. H., et al., 2024).
Ask a Question
Write your own review






