ONLINE INQUIRY

Human Epidermal Melanocytes - juvenile (HEMs-j)
Cat.No.: CSC-C4001X
Species: Human
Source: Epidermis; Skin
Cell Type: Melanocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Epidermal Melanocytes (HEMs-j) from Creative Bioarray are isolated from the healthy human foreskin tissue. The cells are cryopreserved at Passage 2, with each vial containing at least 0.5 x 10^6 cells. Human Epidermal Melanocytes (HEMs-j) are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HEMs-j are guaranteed for further at least 15 population doublings under the conditions provided by Creative Bioarray. Repeated freezing and thawing of cells is not recommended.
The Human Epidermal Melanocytes - Juvenile cell line from Creative Bioarray is isolated from melanocytes in juvenile foetal epidermal tissue. In the skin's epidermal layer, melanocytes transcribe and relocate to the base of the epidermis in embryonic development. Close to the basement membrane, they work with other epidermal cells, including underlying keratinocytes, to keep the skin normal and functioning. Under the microscope, they tend to be dendritic, with cylindrical or oval cell structures and multiple long dendritic projections radiating outwards. The cells contain melanosomes, the organelles that make and store melanin.
HEM cells are widely used in scientific research. They are not only employed to investigate pigmentation disorders and melanocyte senescence, but also to find cures for these conditions, especially malignant melanoma. HEM cells are also used for in vitro wound healing and as model systems for toxicity and irritation experiments, melanoma, skin reaction to ultraviolet light, psoriasis and other skin conditions, and cosmetic research (i.e., skin-whitening agents, skin-protecting agents).
Activation of the p53-TRPM1/miR-211-MMP9 Axis in Melanocytes Treated with Single or Repeated Exposures to UVB
Vitiligo affects 0.5-2% of the global population, manifesting as white patches on the skin due to the loss of melanocytes, and has severe social and psychological impacts on teenagers. UVB-based phototherapy is an effective repigmentation treatment that requires melanocytes to exit their epidermal microenvironment and establish new networks with neighboring keratinocytes. The expression of matrix metallopro-teases (MMPs) in melanocytes appears to be correlated with tissue remodeling or replasticity, but little is known about whether MMP9 affects melanocyte migration in vitiligo repig-mentation. Su et al. investigates the p53-TRPM1/miR-211-MMP9 axis's role in UVB-induced melanocyte migration in vitiligo.
First, the expression profiles of the p53-TRPM1/miR-211-MMP9 axis in primary human epidermal melanocytes (from juvenile foreskin tissues) treated with single or repeated exposures to UVB was examined. The RT-qPCR and western blotting results showed that, in single exposures (0-70 mJ/cm²), p53 and MMP9 levels increased with UVB dose (Fig. 1). TRPM1 levels rose at lower doses (8.75, 17.5 mJ/cm²) but declined at higher doses (35, 70 mJ/cm²). For repeated exposures, cells received cumulative doses of 35 or 70 mJ/cm² (8.75 mJ/cm² per dose at 5-hour intervals). Results (Fig. 2) indicated p53 and MMP9 were elevated, while TRPM1 decreased after multiple UVB exposures compared to controls. These results demonstrated that the p53-TRPM1/miR-211-MMP9 axis is significantly activated in melanocytes by single and by repeated exposures to UVB.
 Fig. 1. Expression profiles of the p53-TRPM1/miR-211-MMP9 axis in melanocytes treated with a single exposure to UVB (Su M, Miao F, et al., 2020).
Fig. 1. Expression profiles of the p53-TRPM1/miR-211-MMP9 axis in melanocytes treated with a single exposure to UVB (Su M, Miao F, et al., 2020).
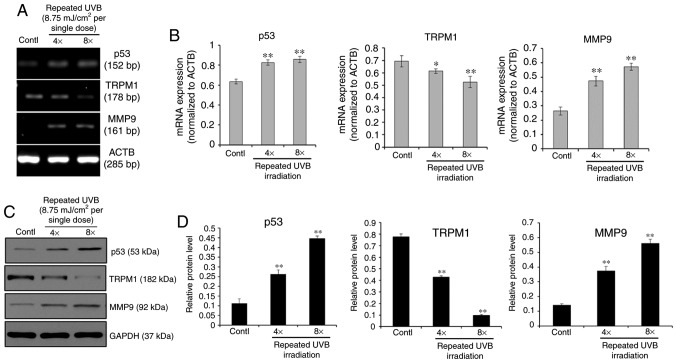 Fig. 2. Expression profiles of the p53-TRPM1/miR-211-MMP9 axis in melanocytes treated with repeated exposures to UVB (cumulative doses of 35 (4X) or 70 (8X) mJ/cm², given at 8.75 mJ/cm² per exposure in 5 h intervals for a total of 4 or 8 times) (Su M, Miao F, et al., 2020).
Fig. 2. Expression profiles of the p53-TRPM1/miR-211-MMP9 axis in melanocytes treated with repeated exposures to UVB (cumulative doses of 35 (4X) or 70 (8X) mJ/cm², given at 8.75 mJ/cm² per exposure in 5 h intervals for a total of 4 or 8 times) (Su M, Miao F, et al., 2020).
UVB-exposed MCs are Actively Motile and Release the sPmel17
Vitiligo is a chronic acquired autoimmune disease characterized by skin depigmentation due to loss of melanocytes. Although phototherapy such as NB-UVB is an effective treatment. However, patient responsiveness to this phototherapy varies widely and long-term treatment reduces compliance. It is also challenging to predict when and where hyperpigmentation will occur in UVB-treated lesions.
In patients with vitiligo, the depigmented epidermis is derived from undamaged hair follicles and/or functional melanocytes (MC) migrating from the surrounding area. Hu et al. defined whether MCs (primary Epidermal Melanocytes – juvenile) secrete a secreted form of Pmel17 (sPmel17) protein in the face of UVB, thus weakening KCs' (human epidermal keratinocytes) cell-cell adhesions and permitting MCs to migrate to sites where there were no MCs. They compared mRNA expression of genes involved in melanogenesis (MITF, TYR, TYRP1, DCT, and Pmel17) and the migrating gene MCAM (melanoma cell adhesion molecule) under and without 30 mJ/cm² UVB. The qRT-PCR result revealed that UVB increases the expression of all genes related to melanogenesis and MCAM in transcriptional levels (Fig. 3a). Similar protein levels were found with western blotting (Fig. 3b). Interestingly, an increased level of sPmel17 protein was seen in the CM from UVB-exposed MCs (Fig. 3c). Moreover, Transwell assay results indicated that UVB significantly promotes MC migration on a collagen IV-coated surface (Fig. 3d). These findings suggest that UVB-exposed MCs achieve an active motile phenotype and release sPmel17 protein into the extracellular environment.
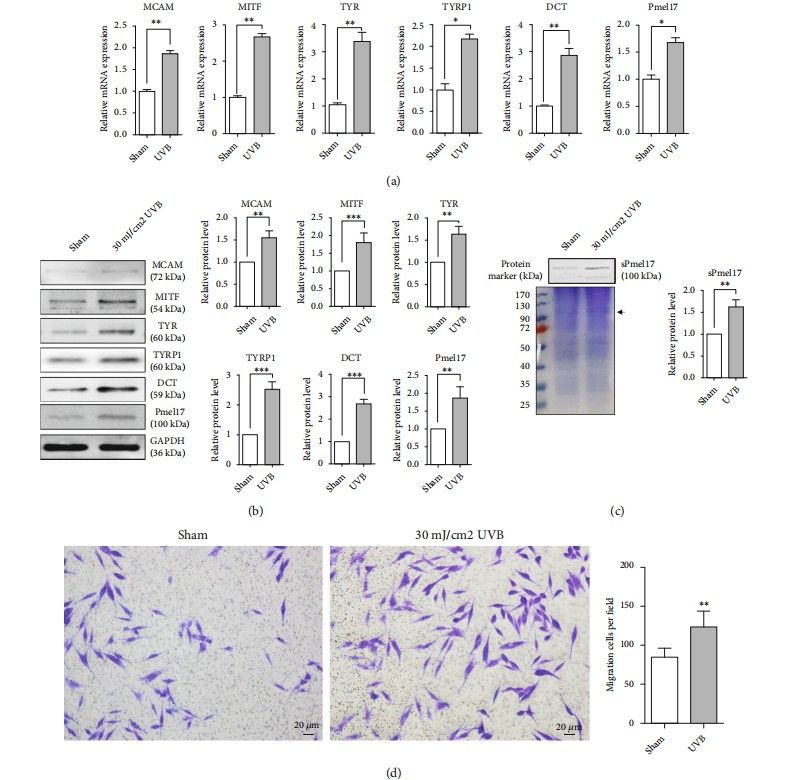 Fig. 3. MCs achieve the phenotypes of melanogenesis and migration following UVB exposure. Primary human epidermal MCs wereisolated from juvenile foreskin tissues, treated with or without 30 mJ/cm² UVB and then cultured for one additional day (Hu S H, Jiang S, et al., 2022).
Fig. 3. MCs achieve the phenotypes of melanogenesis and migration following UVB exposure. Primary human epidermal MCs wereisolated from juvenile foreskin tissues, treated with or without 30 mJ/cm² UVB and then cultured for one additional day (Hu S H, Jiang S, et al., 2022).
Ask a Question
Write your own review


