ONLINE INQUIRY

Human Keratinocytes - juvenile (HKs-j)
Cat.No.: CSC-C4003X
Species: Human
Source: Epidermis; Skin
Cell Type: Keratinocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Creative Bioarray provides Human Keratinocytes - juvenile (HKs-j) isolated from the dermis of juvenile foreskin. These cells are cryopreserved at Passage 2 and shipped frozen, with each vial containing at least 0.5 x 10^6 cells. HKs-j are free from HIV-1, HBV, HCV, mycoplasma, bacteria, yeast, and fungi, and are guaranteed for at least 15 population doublings under the conditions provided by Creative Bioarray. Repeated freezing and thawing of cells is not recommended.
Creative Bioarray's Human Keratinocytes - juvenile (HKs-j) cell line is derived from the foreskin dermis of juvenile and has been proven as a stable cell line under in vitro culture conditions. They reside in the epidermal layer of the skin and are one of the major cell types of the epidermis. Between the innermost layer (basal layer) and outermost layer (stratum corneum), keratinocytes proliferate, differentiate and die. As they differentiate, they continue to produce more and more keratin, a very abundant structural protein that is what makes the skin stiff and protective.
This cell line has outstanding proliferative potential - particularly for cells of basal lamina. They can quickly mitose the cells under an optimal in vitro culture system. Meanwhile, this cell line does have a well-established differentiation program. As cells move up from the bottom and out to the upper layers, they gradually go through a set of elaborate differentiation steps. In the process, gene expression inside the cells is drastically altered, while expression of keratin changes gradually, and so does its type and structure. As a result, they are ubiquitously used to investigate basic biology of the skin, such as cell proliferation, differentiation, apoptosis and immune response. These cells are also ideal candidates for studying diseases of the skin such as psoriasis and skin cancer, to help unravel the causes of the conditions and identify potential cures. Furthermore, they serve as toxicological testing specimens for skin irritation and corrosion.
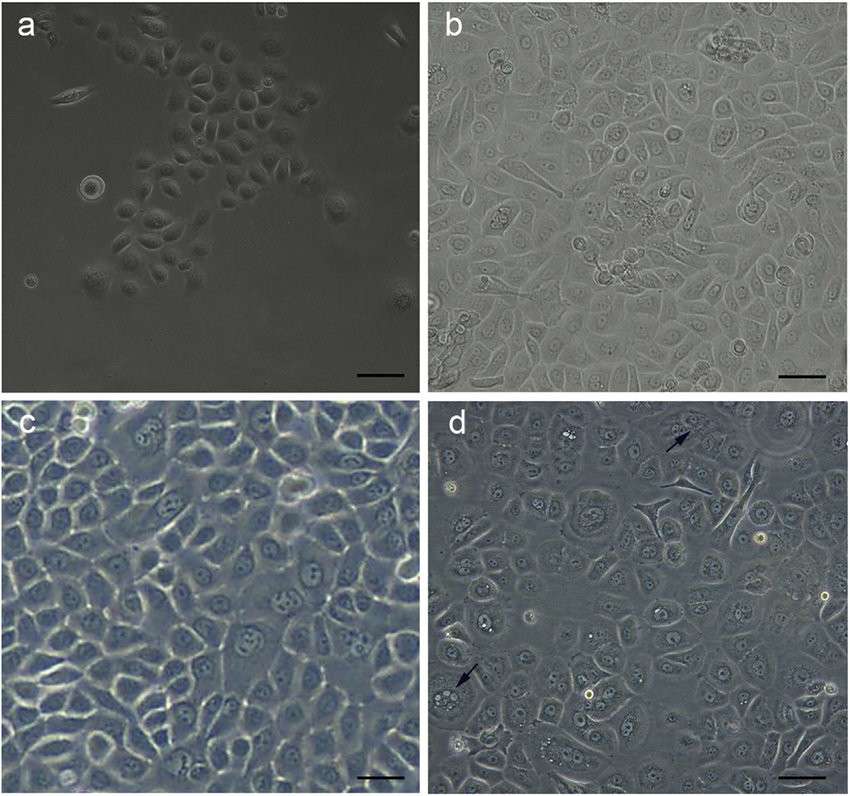 Fig. 1. Morphology of foreskin-derived keratinocytes (a: passage 0; b: passage 3; c: passage 5, d: passage 7) (Akhavan-Tavakoli M, Fard M, et al., 2017).
Fig. 1. Morphology of foreskin-derived keratinocytes (a: passage 0; b: passage 3; c: passage 5, d: passage 7) (Akhavan-Tavakoli M, Fard M, et al., 2017).
Orf Virus Rapidly Induces Cell Death of Keratinocytes but not Fibroblasts
The skin serves as a major barrier to pathogens while also being a manifestation site for diseases such as viral infections. Keratinocytes in the epidermis and dermal fibroblasts are crucial immune activators. Orf virus (ORFV), a pathogen of small ruminants, can infect human skin, leading to pustular dermatitis. Schneider et al. explored the effects of ORFV on human primary keratinocytes (KC) and fibroblasts (FB).
Results indicated that ORFV strain B029 infection rapidly induced morphological changes in primary human KC, with 40% of KC detaching or becoming rounded within 6 hours (Fig. 1A). No such changes were seen in FB or KOP cells early, but they became pronounced after 96 hours, along with more pronounced KC morphological changes (Fig. 1A). Lactate dehydrogenase (LDH) release was analyzed since LDH is only released from damaged cells. The results showed increased LDH release indicating ORFV-induced KC cell death, while FB or KOP LDH levels did not significantly increase (Fig. 1B). Cytosine arabinoside (AraC) inhibits DNA synthesis and thus the expression of late but not early ORFV genes. Cycloheximide (CHX) inhibits protein synthesis. Adding AraC didn't prevent ORFV-induced cell death, but CHX treatment reduced it (Fig. 1C), suggesting early gene products as inducers of KC cell death. Orf virus encodes both apoptosis inhibitors and pro-apoptotic proteins. They examined whether cell death in ORFV-infected keratinocytes (KC), fibroblasts (FB), and KOP cells was due to apoptosis or necrosis using annexin V and propidium iodide (PI) staining. ORFV infection rapidly induced cell death in KC, primarily characterized by an increase in late apoptotic/necrotic cells from 6 to 24 hours post-infection (hpi) (Fig. 1D, E, F). In FB, a similar increase was only observed at 24 hpi, and in KOP cells, ORFV weakly induced necrosis. Staurosporine, a strong apoptosis inducer, confirmed the high rates of cell death. These findings suggest that ORFV quickly triggers cell death in KC, though further research is needed to clarify the exact mechanism.
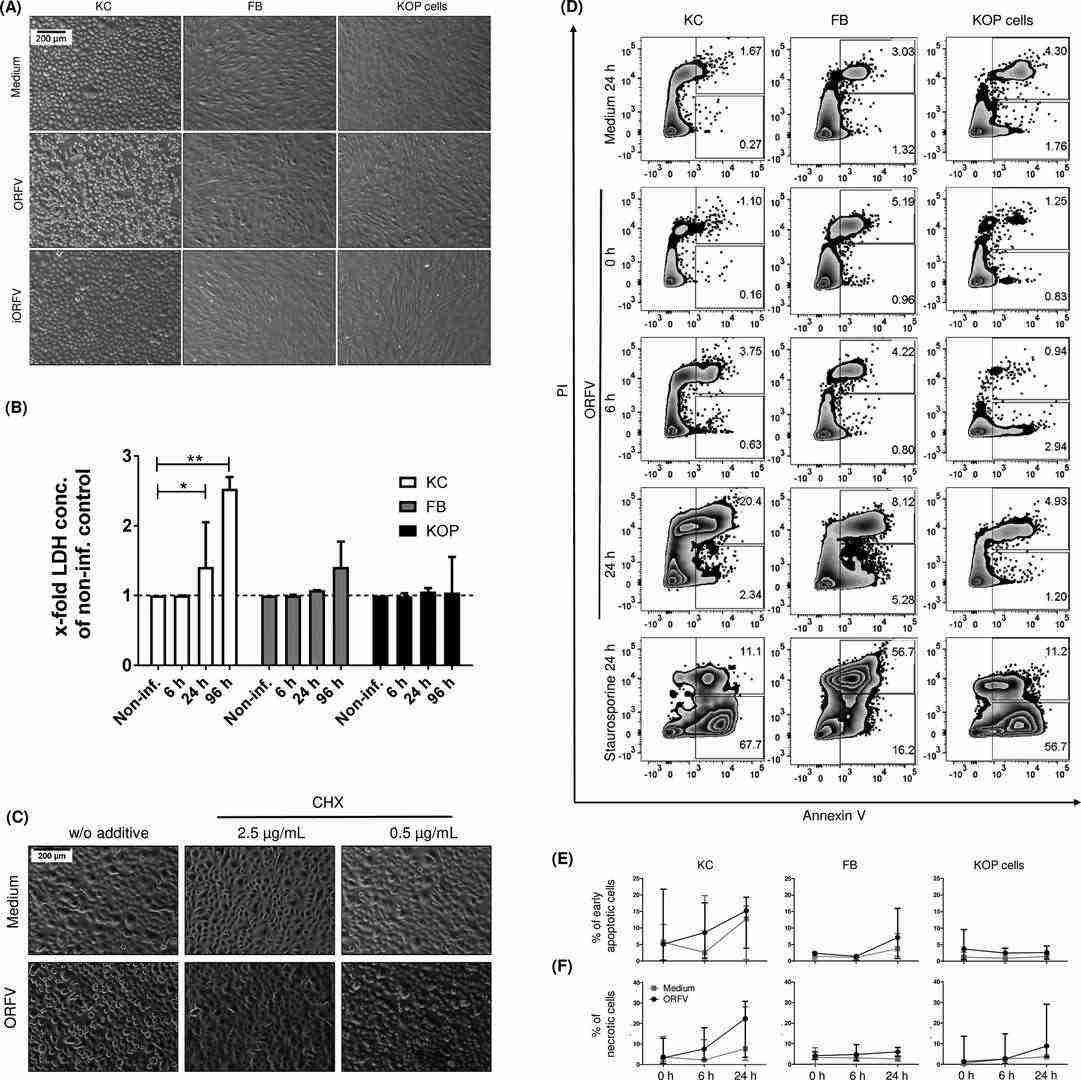 Fig. 1. Orf virus (ORFV) induces rapid cell death in human primary keratinocytes (KC) (Schneider L E., Protschka M., et al., 2018).
Fig. 1. Orf virus (ORFV) induces rapid cell death in human primary keratinocytes (KC) (Schneider L E., Protschka M., et al., 2018).
miR-497-5p's Control Differentiation and Proliferation of Keratinocytes Exposure to Sulfur Mustard
Sulfur mustard (SM) is a banned cytotoxic chemical warfare agent that causes severe skin damage and delayed wound healing. SM exposure leads to the alkylation of DNA and proteins in the skin, with precise pathomechanisms remaining unclear. The proliferation and differentiation of epidermal keratinocytes is necessary for wound healing. Previous research has observed miRNA dysregulation in SM-treated keratinocytes, but it remains unclear how targeted therapy would be executed.
Egea et al. used RNA-seq technology to analyze SM's genome-wide effects on mRNA and miRNA in human keratinocytes, identifying key miRNAs affecting skin cell function and wound healing, particularly miR-497-5p and its target survivin. Experiments showed SM triggers differentiation and inhibits keratinocyte proliferation, they hypothesizing miRNAs might be involved in SM-mediated dysregulation of differentiation and proliferation in keratinocytes. They probed the effects of miRNAs on keratinocyte differentiation and growth, identifying four miRNAs that were significantly elevated in SM-treated NHEK: miR-7-5p, miR-34c-5p, miR-129-5p and miR-497-5p. The overexpression of miR-34c-5p, miR-129-5p and miR-497-5p boosted keratin 1 and decreased Ki-67 in NHEK (Fig. 2A, B), which suggests that they encourage differentiation and suppress proliferation. The study highlighted miR-497-5p's role: it enhanced keratin 5, 16, and involucrin expression in treated NHEK, indicating a contribution to SM-induced maturation processes (Fig. 2D). Suppressing miR-497 encouraged proliferation in normal and SM-exposed cells (Fig. 2E), underscoring its potential in treating SM-induced skin deficiencies.
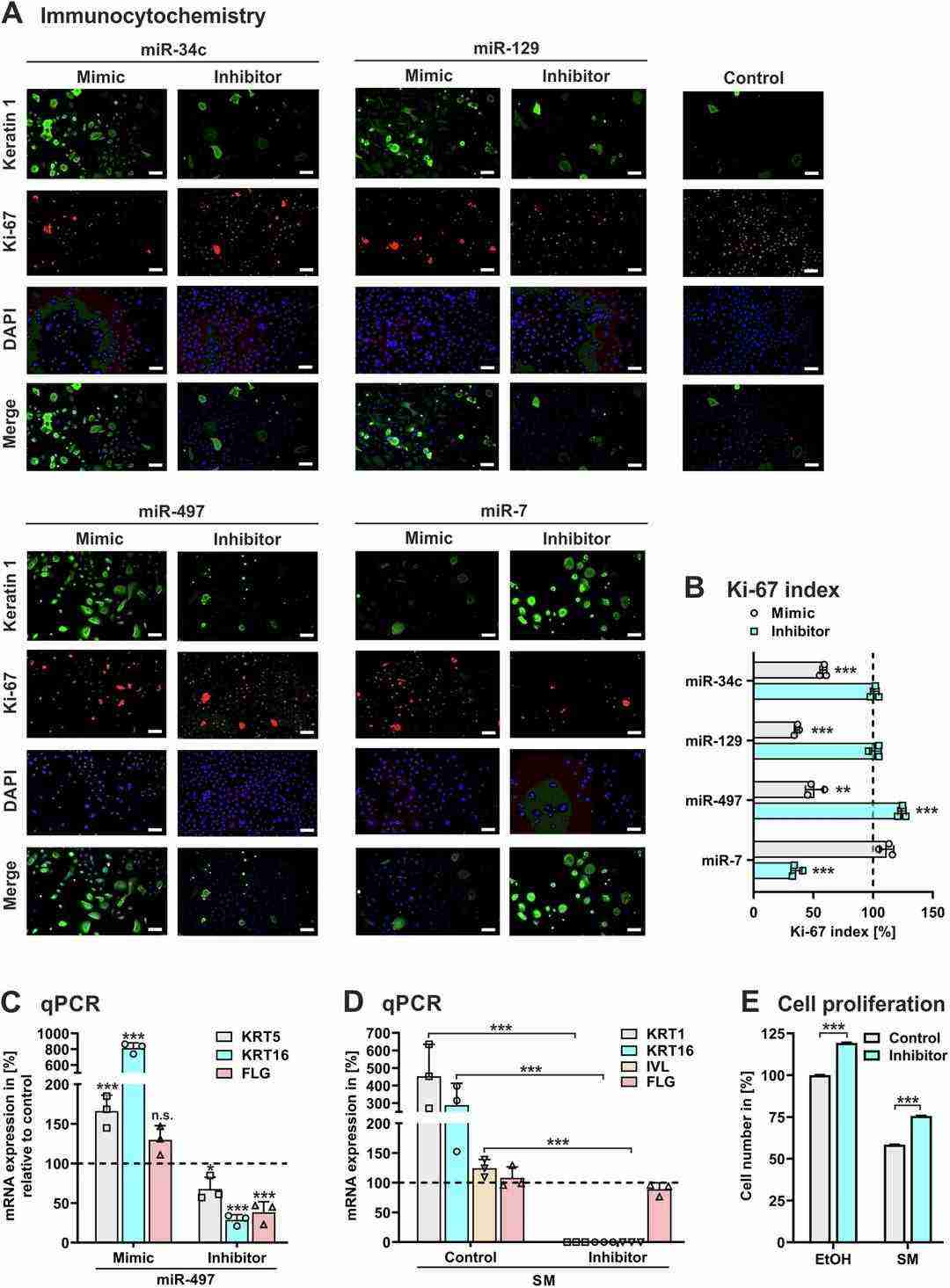 Fig. 2. NHEK were transfected with synthetic LNA miRCURY miRNA (mimic), LNA miRCURY inhibitor of miRNA (inhibitor), or non-specific miRNA (control) (Egea V, Lutterberg k, et al., 2024).
Fig. 2. NHEK were transfected with synthetic LNA miRCURY miRNA (mimic), LNA miRCURY inhibitor of miRNA (inhibitor), or non-specific miRNA (control) (Egea V, Lutterberg k, et al., 2024).
Ask a Question
Write your own review


