ONLINE INQUIRY
Canine Embryonic Fibroblasts
Cat.No.: CSC-C4807L
Species: Dog
Source: Embryo
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Canine embryonic fibroblasts are typically sourced from embryonic tissue and are isolated during early embryonic stages. These cells demonstrate significant pluripotency capabilities that enable them to remain undifferentiated or differentiate into multiple cell types when cultured in vitro.
Canine embryonic fibroblasts act as widely utilized "feeder layers" in research while supporting other stem cell growth. Canine embryonic fibroblasts produce multiple cytokines including fibroblast growth factor (FGF) and interleukins (IL) which promote stem cell proliferation while preventing differentiation. Researchers employ these cells as model systems to test gene editing technologies including CRISPR/Cas9 which helps understand gene functions and disease mechanisms. Moreover, their pluripotency makes them valuable tools for testing new drugs and determining substance toxicity.
Panobinostat Effectively Inhibits Endogenous Histone Deacetylase and Promotes Chromatin Acetylation
While canine iPSCs hold potential for regenerative medicine, current reprogramming protocols are insufficient. Histone modifications are crucial for chromatin remodeling required for iPSC induction. Moshref et al. investigated the potential of histone deacetylase (HDAC) inhibition, specifically using panobinostat and vitamin C, to enhance reprogramming efficiency by increasing chromatin accessibility in canine embryonic fibroblasts (CEF), thus seeking to improve iPSC derivation protocols for canine cells.
After identifying CEF-specific cytotoxic levels, they assessed the HDAC inhibitory effects of various compounds. Panobinostat (1, 5, 10 nM) and TSA (12.5, 25, 50 nM) significantly reduced HDAC activity, while VPA and sodium butyrate did not (Fig. 1). They focused on H3K9 and H3K27 acetylation due to their reprogramming significance. H3K27ac marks active enhancers, and H3K9ac promotes gene transcription. Both TSA and panobinostat increased histone acetylation in CEF at all concentrations tested (Fig. 2). Overall, although TSA inhibited HDAC and enhanced acetylation, only panobinostat at 1 nM effectively inhibited HDAC and increased acetylation without affecting cell proliferation.
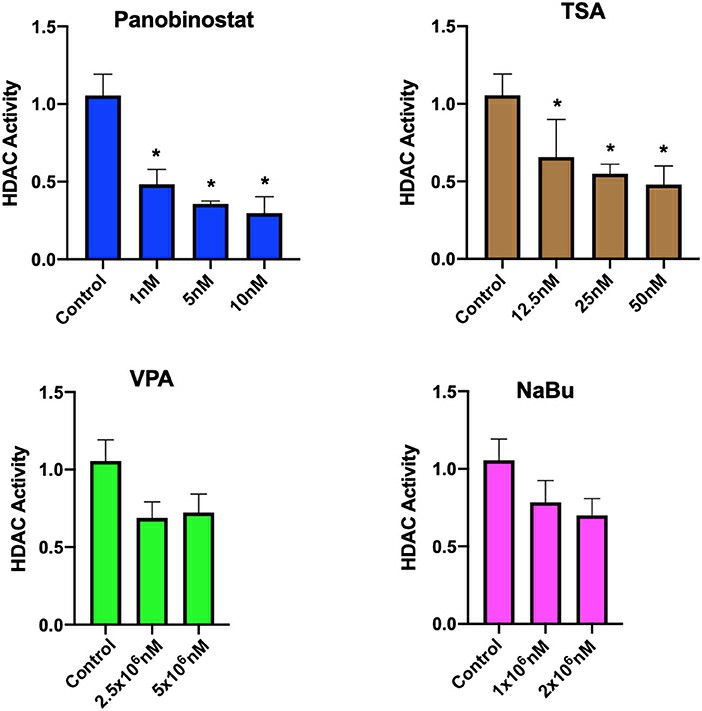 Fig. 1. Assessment of HDAC activity in CEF upon HDACi treatment (Moshref M, Questa M, et al., 2021).
Fig. 1. Assessment of HDAC activity in CEF upon HDACi treatment (Moshref M, Questa M, et al., 2021).
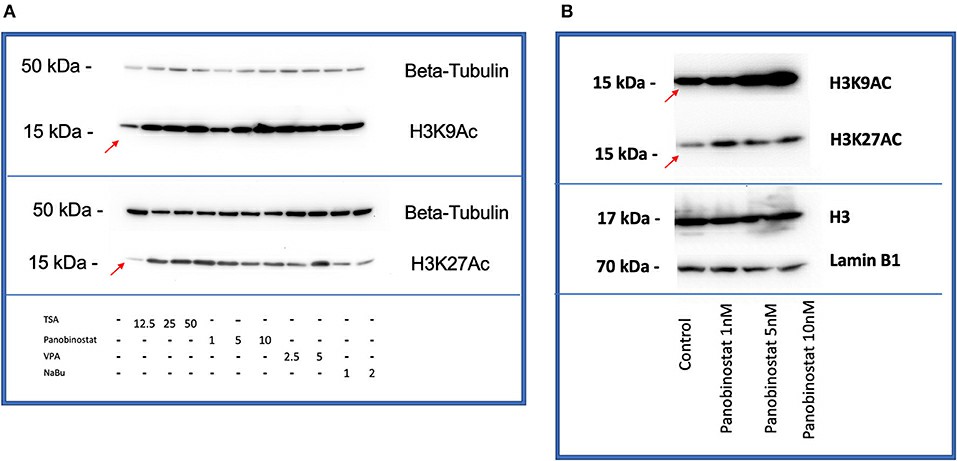 Fig. 2. HDACi effectively increases H3K9 and H3K27 histone acetylation in CEF (Moshref M, Questa M, et al., 2021).
Fig. 2. HDACi effectively increases H3K9 and H3K27 histone acetylation in CEF (Moshref M, Questa M, et al., 2021).
Canine Embryonic Fibroblasts but not Adult Stromal Cells can be Reprogrammed to ciPSC
Naturally occurring diseases in dogs provide a valuable model for regenerative medicine. Although embryonic fibroblasts can be reprogrammed to iPSCs, adult stromal cells resist due to potential epigenetic barriers. Questa et al. aims to explore chromatin accessibility during reprogramming of canine cells using ATAC-seq, identify candidate genes as reprogramming barriers, and propose new canine-specific methods to enhance iPSC derivation.
They transduced four Yamanaka factors (OCT4, SOX2, KLF4, and MYC; OSKM) into low passage CEF, cASC, and CDF. CEF reprogrammed into ciPSC colonies exhibited stem cell-like morphology and high alkaline phosphatase activity (Fig. 3A). These colonies expressed pluripotency genes OCT4, NANOG, and SOX2 (Fig. 3B and C) and differentiated into cells of all three germ layers (Fig. 3D). ciPSC later silenced transgene expression. They also used an OCT4-eGFP reporter system to confirm genuine reprogramming in CEF. eGFP expression indicated activation of endogenous OCT4, visible from day 4 PT to later passages (Fig. 3E). The luciferase assay showed that the OCT4 locus is controlled by the proximal enhancer, indicating a primed state (Fig. 3F). However, cASC and CDF did not form stable colonies.
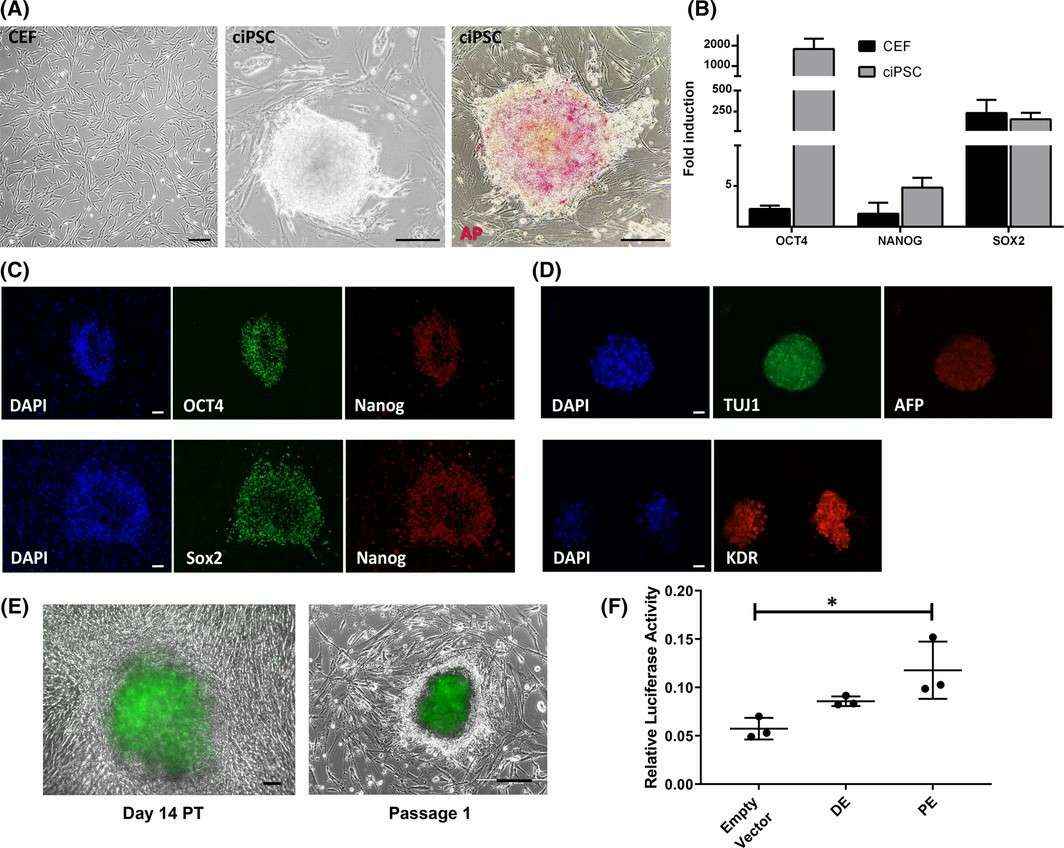 Fig. 3. Generation of ciPSC and regulation of endogenous OCT4 expression in ciPSC (Questa M, Moshref M, et al., 2021).
Fig. 3. Generation of ciPSC and regulation of endogenous OCT4 expression in ciPSC (Questa M, Moshref M, et al., 2021).
Ask a Question
Write your own review


