ONLINE INQUIRY
Human Small Intestinal Epithelial Cells
Cat.No.: CSC-C9229J
Species: Human
Source: Small Intestine; Intestine
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Human small intestinal epithelial cells (IECs) are highly specialized cells that line the lumen of the small intestine, playing key roles in nutrient absorption, barrier function, and immune regulation. These cells form a monolayer epithelium organized into crypt-villus structures, which maximize the surface area for efficient nutrient uptake. Functionally, IECs are responsible for the digestion and absorption of carbohydrates, proteins, and fats by expressing specific transporters and enzymes, such as disaccharidases and peptidases. Additionally, they maintain the integrity of the intestinal barrier via tight junctions, preventing the translocation of pathogens and toxins. IECs also contribute to mucosal immunity by secreting antimicrobial peptides (e.g., defensins from Paneth cells), mucus (from goblet cells), and cytokines, while interacting with immune cells to modulate local and systemic immune responses. Some of the most severe pathological conditions, such as inflammatory bowel disease (IBD), are known to be associated with alterations of the normal growth and function of the intestinal epithelium. It is therefore of interest to investigate the physiology and pathophysiology of these cells and, not surprisingly, a large number of scientists in research fields such as intestinal physiology, intestinal immunology and cancer genesis are interested in the study of these cells.
Advantages of using human small intestinal epithelial cells include their relevance to human biology, the ability to mimic in vivo conditions, and the versatility in experimental setups. However, challenges remain, such as maintaining long-term viability and functional stability in vitro. Advances in 3D culture systems and organ-on-a-chip technologies are addressing these limitations, further enhancing their utility in biomedical research.
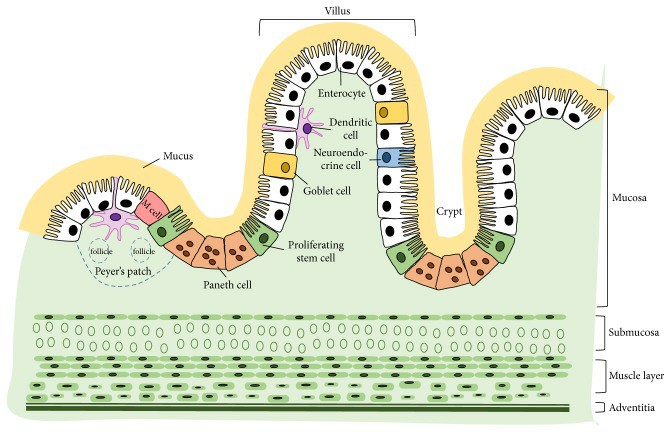 Fig. 1. Cross-sectional structure of small intestine and the major cell constituents of epithelium (Kong, Shanshan, et al. 2018).
Fig. 1. Cross-sectional structure of small intestine and the major cell constituents of epithelium (Kong, Shanshan, et al. 2018).
Anti-Inflammatory Effects in Intestinal Epithelial Cells
Given growing understanding of the importance of peripheral inflammation, gut-health and the gut-brain axis on cognitive health, the effects of S. officinalis extract were assessed in human small intestinal epithelial cells as a model of the intestinal barrier.
Figures 1 and 2 summarize the levels of cytokines and chemokines released into apical and basolateral cell media respectively by human small intestinal epithelial cells incubated with LPS and S. officinalis extract for 24 h. Compared to the control group, the LPS treatment caused a significant increase in the release of all eight of the protein markers tested in the apical media (Fig. 1) and all but CRP and VCAM-1 in the basolateral media (Fig. 2), which supports successful induction of pro-inflammatory response by the LPS treatment.
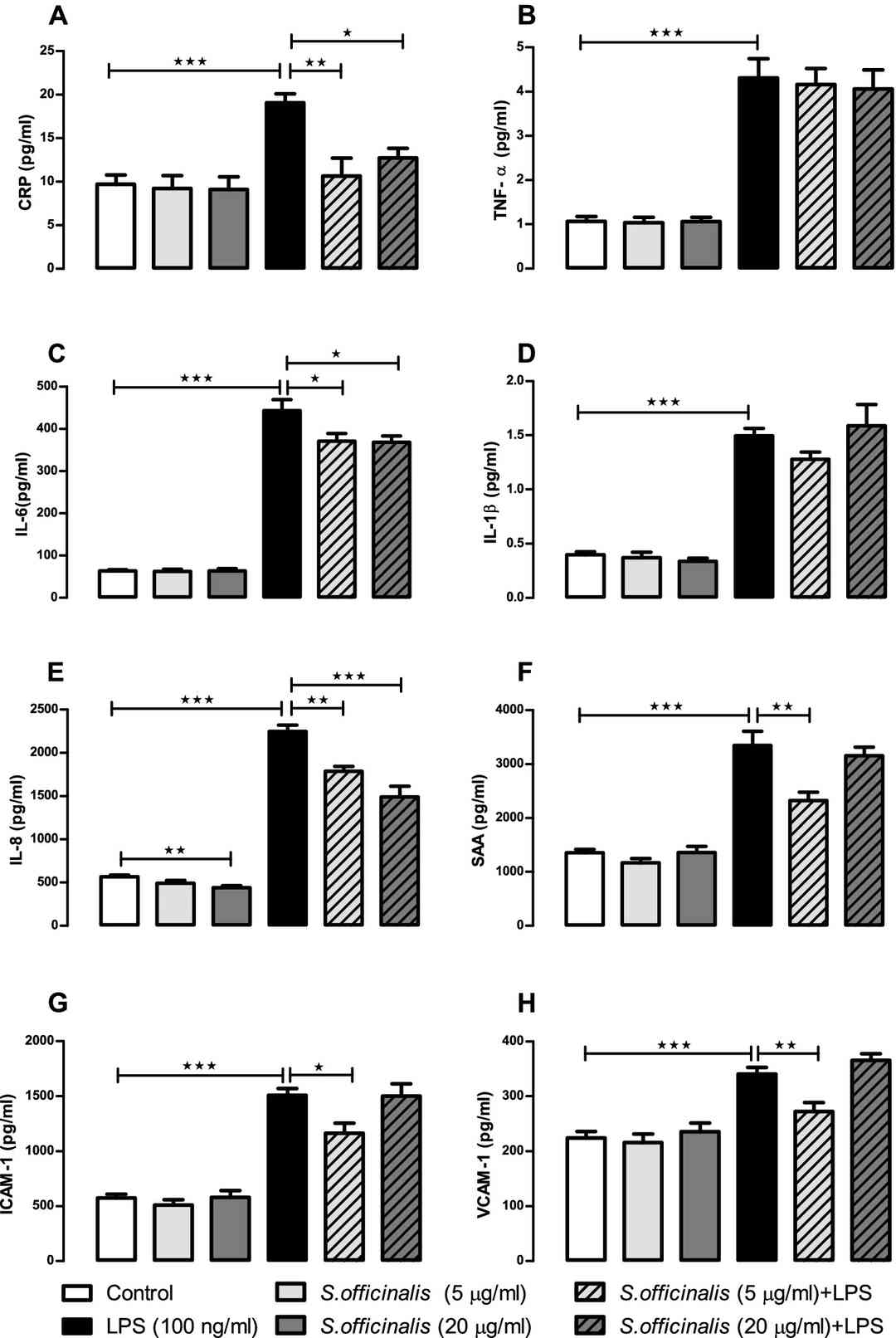 Fig. 1. S. officinalis extracts attenuation of cytokine and chemokine release by human small intestinal epithelial cells in apical media after 24-h treatment with LPS (Margetts, Gemma, et al. 2022).
Fig. 1. S. officinalis extracts attenuation of cytokine and chemokine release by human small intestinal epithelial cells in apical media after 24-h treatment with LPS (Margetts, Gemma, et al. 2022).
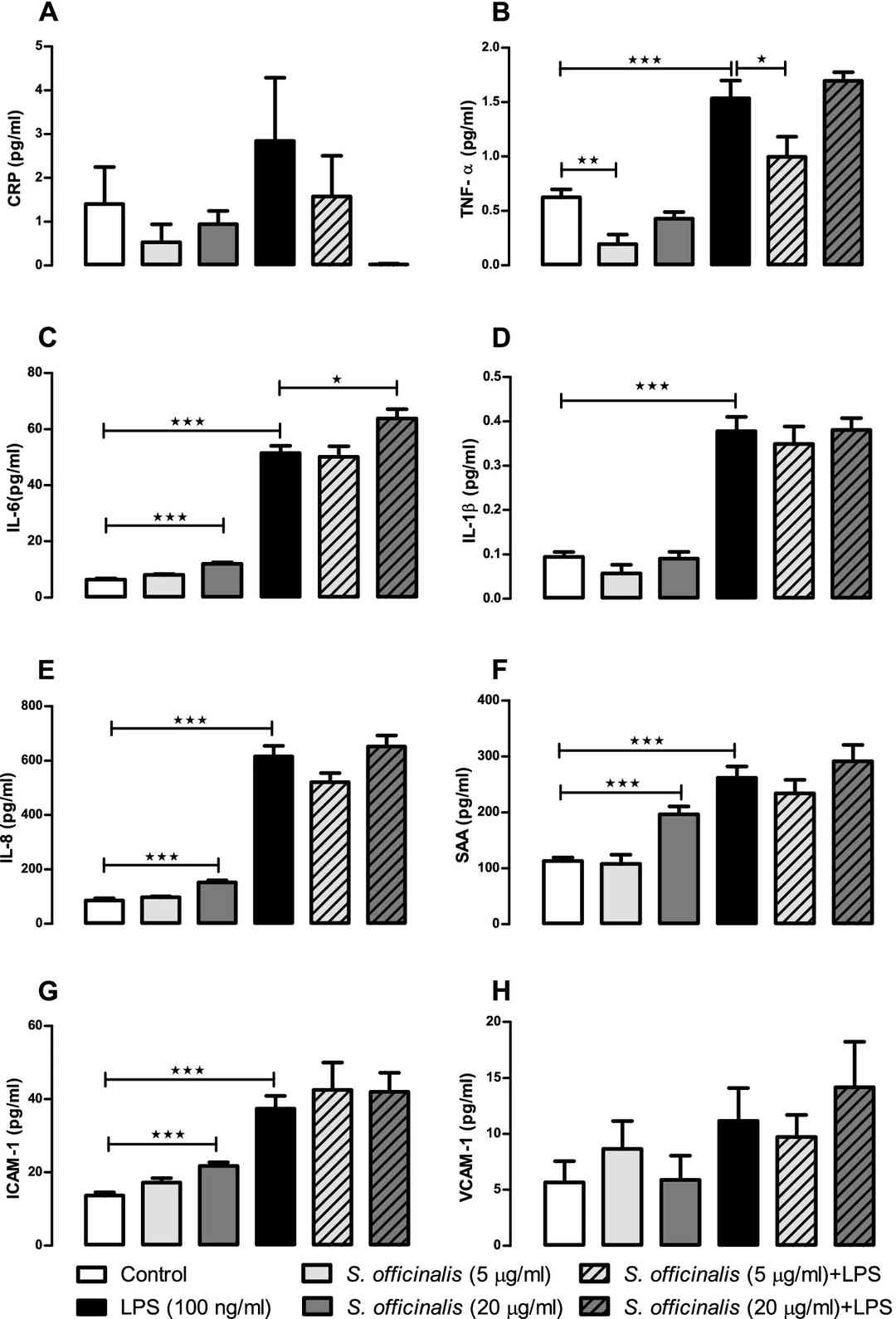 Fig. 2. S. officinalis extracts attenuation of cytokine and chemokine release by human small intestinal epithelial cells in basolateral media after 24-h treatment with LPS (Margetts, Gemma, et al. 2022).
Fig. 2. S. officinalis extracts attenuation of cytokine and chemokine release by human small intestinal epithelial cells in basolateral media after 24-h treatment with LPS (Margetts, Gemma, et al. 2022).
Characterization of Conditions for The Primary Culture of Human Small Intestinal Epithelial Cells
Intestinal epithelial cells (IECs) were cultured on collagen membranes in a 12-well tissue culture cluster, with Lamina propria (LP) cells and allogeneic Epstein-Barr Virus (EBV)-transformed B lymphocytes (allo-B cells) underneath, in the well.
Primary human IECs in culture did not form confluent monolayers but cells did maintain contact with other cells in small sheets. Individual cells did maintain their cellular polarity but the cells were not all aligned on the membrane.
Initial experiments of IECs cultured above the LP fraction and allo-B cells (E/LP + B), showed that cells cultured for one day maintained their columnar/cuboid morphology and polarity (Fig. 3a, b). Morphology was further maintained when cells were cultured for 2 days (Fig. 3c, d) and for 7 days (Fig. 3f, g). All IECs expressed the human epithelial antigen as detected by Ber-EP4 antibody (Fig. 3f, g), and there was no evidence of contamination by fibroblasts.
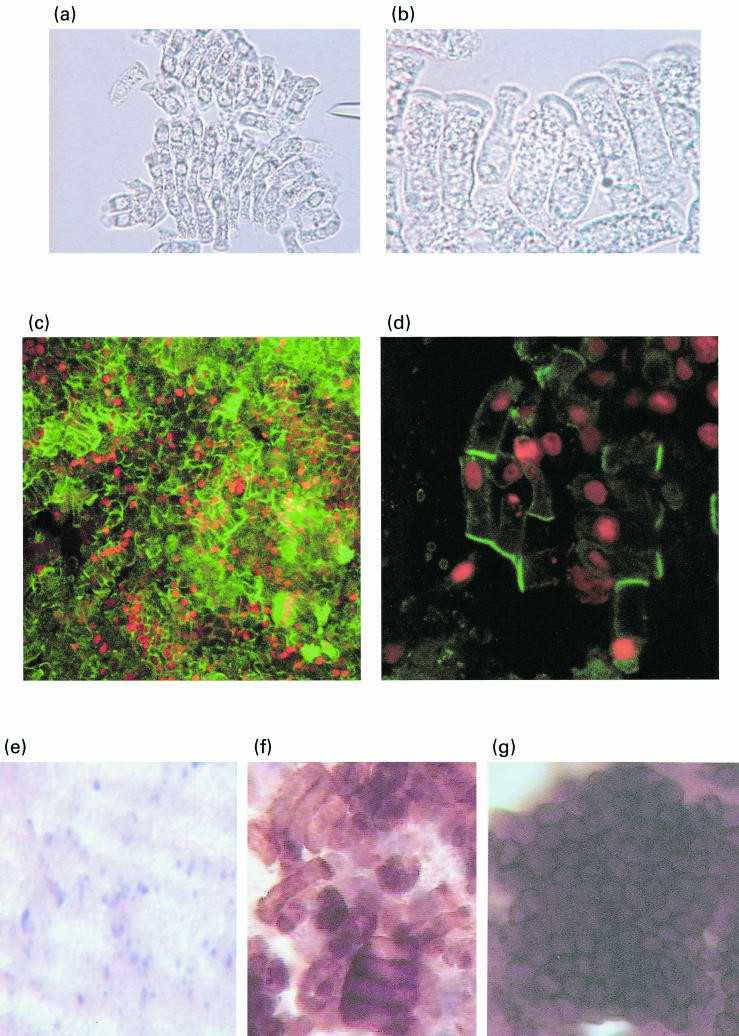 Fig. 3. Culture of epithelial cells above LP fraction and allo-B cells in the culture system (Aldhous, M. C., et al. 2001).
Fig. 3. Culture of epithelial cells above LP fraction and allo-B cells in the culture system (Aldhous, M. C., et al. 2001).
Our cells can be handled at biosafety level 1, but caution is always recommended when handling human tissue derived products. Hope this helps.
Ask a Question
Write your own review


