ONLINE INQUIRY

Human Skeletal Muscle Cells (DMD)
Cat.No.: CSC-C3604
Species: Human
Source: Skeletal Muscle
Cell Type: Skeletal Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
DMD is a recessive X-linked form of muscular dystrophy characterized by rapid progression of muscle degeneration.
Origin: Human
Disease: Duchenne Muscular Dystrophy (DMD)
Application: These cells can be used for various types of in vitro, in vivo, or regenerative medicine studies in normal or diseased systems and in muscle development studies.
Note: Never can cells be kept at -20 °C.
Duchenne muscular dystrophy (DMD) is a fatal X-linked recessive disease that affects 1 in 5.000 newborn males. It is typically caused by deletions disrupting the open reading frame of the DMD gene, resulting in a lack of dystrophin protein. Dystrophin plays a major role in membrane stabilization during muscle contraction, linking the actin cytoskeleton to the sarcolemma and also contributes to extracellular signalling. The lack of dystrophin in the muscles of DMD patients leads to progressive muscle wasting and degeneration. Currently, there is no curative treatment for DMD.
In vitro cell models are particularly useful for evaluating the efficiency of novel therapies for DMD. However, only a few human immortalized muscle cell lines derived from DMD patients are currently available. Due to the wide spectrum of DMD mutations and the difficulties to obtain DMD patient muscle biopsies, DMD-myoblasts models would provide a powerful resource for in vitro drug screening and study disease rescue mechanisms.
Creative Bioarray offers single-donor Primary Human Skeletal Muscle Cells and Skeletal Muscle Myoblasts, characterized by age, gender, race, and BMI with specially formulated media that is optimized to make cell culture growth, differentiation, and cryopreservation as reliable as possible in your lab.
Characterizing Primary Donor Cells from Duchenne Muscular Dystrophy (DMD) Patients
The process of donor-specific myoblast selection and cell expansion is illustrated in Fig. 1A. In all, cells from 4 donors were selected with 2 coming from DMD patients and the other 2 coming from non-DMD individuals.
For further characterization, the cells were differentiated and stained for Myosin heavy chain (MHC) to identify myotubes (Fig. 1B). Cell images were then used to extract the myotube area (MHC-positive area), and nuclei count (Fig. 1C), which was used to compute the fusion index. As expected, the fusion index for the DMD donors (DMD #1 and DMD #4) was significantly lower compared to non-DMD donors (Non-DMD #1 and Non-DMD #3).
Dystrophin expression was characterized by immunofluorescence (IF) and simple western blot (Fig. 1D-1F). Nuclei and myotubes were identified using Hoechst and Troponin T respectively. The DMD donors have very low to no expression of Dystrophin compared to the non-DMD donors.
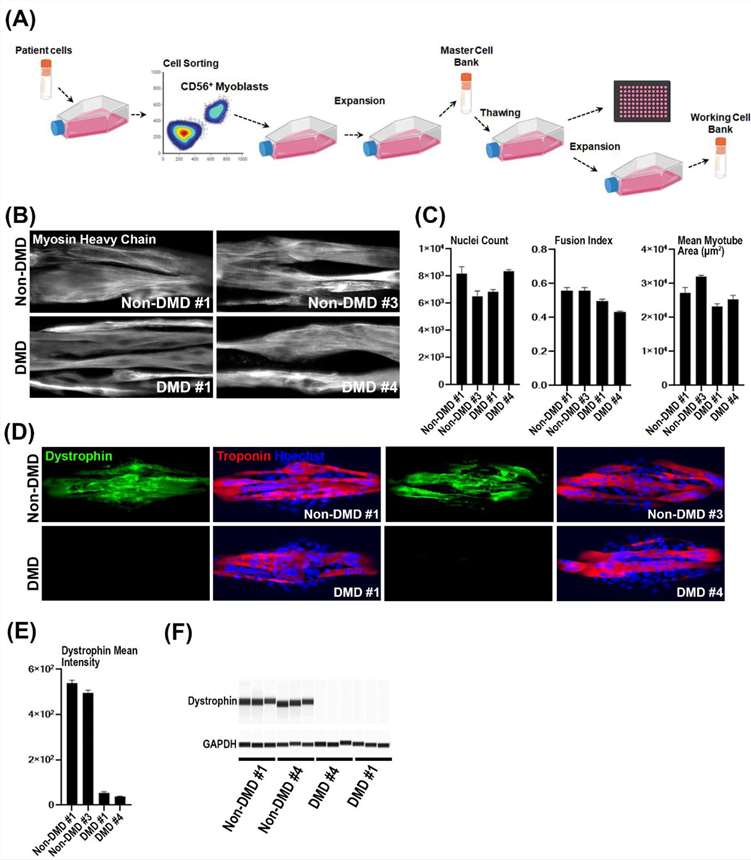 Fig. 1. Donor selection, myotube quantification and dystrophin expression validation (Hariharan, Santosh, et al. 2023).
Fig. 1. Donor selection, myotube quantification and dystrophin expression validation (Hariharan, Santosh, et al. 2023).
Intracellular CD56 Intensity Per Nuclei Does Not Correlate with The Fusion Index
Skeletal muscle-derived cells (SMDC) hold tremendous potential for replenishing dysfunctional muscle lost due to disease or trauma. Predicting the therapeutic potency of SMDC in vitro prior to implantation is key to developing successful therapeutics in regenerative medicine and reducing implementation costs. The primary aim of the study was thus precisely to evaluate the applicability of multi-parametric imaging-based phenotypic characterisation to distinguish the myogenic potency of SMDC obtained from different donors.
CD56 is a reliable marker for myoblasts among cultured cells from skeletal muscle and it was reasoned that its level of expression could reflect the fusion index in cells derived from different donors. However, the mean intracellular CD56 intensity per nuclei did not correlate with the fusion index after 5 days of differentiation and the range of CD56 intracellular staining intensity between donors was small (Fig. 2).
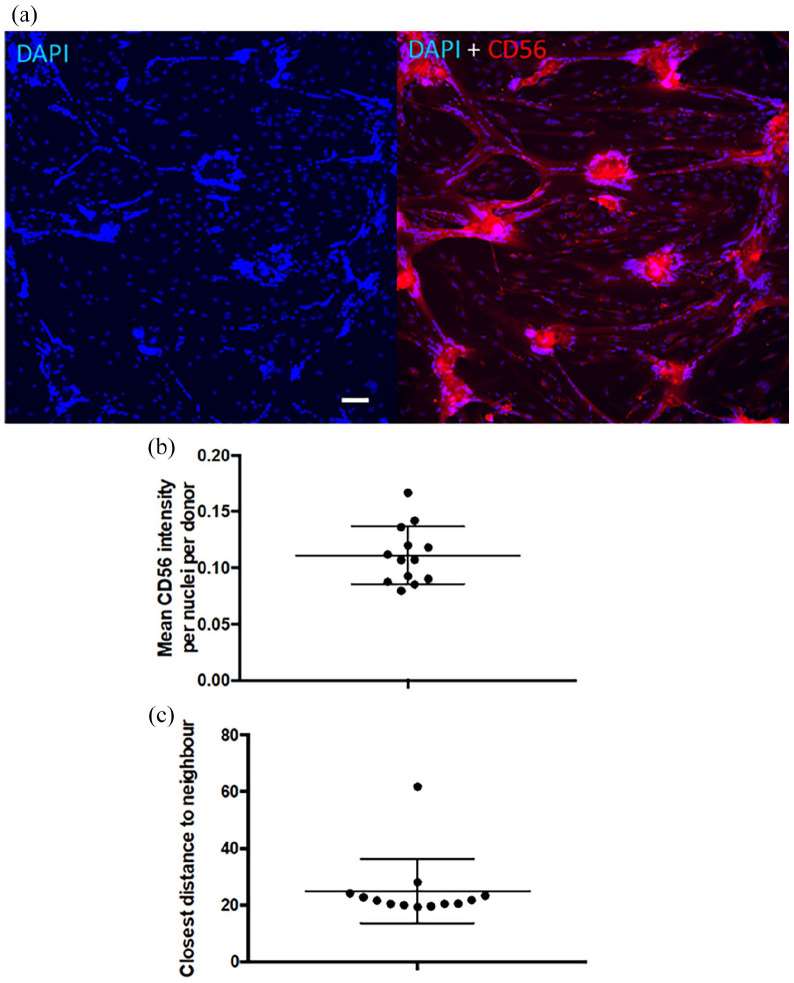 Fig. 2. CD56 intracellular expression (Desprez, Charlotte, et al. 2023).
Fig. 2. CD56 intracellular expression (Desprez, Charlotte, et al. 2023).
Population doubling is 80-120 or up to 12 passages when cultured at 60% to 70% confluent in Creative Bioarray culture system.
Ask a Question
Write your own review
- You May Also Need

