ONLINE INQUIRY

Human Skeletal Muscle Myoblasts (HSkMM)
Cat.No.: CSC-7713W
Species: Human
Source: Skeletal Muscle
Cell Type: Myoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Age- or injury-induced muscle weakness leading to frailty is a major public health problem that is predicted to escalate in the future as the number and proportion of older adults increase in the general population. There is an unmet need for therapeutic strategies to slow the effects of aging on muscle function in frail elderly so as to maintain or improve their quality of life. Identifying appropriate therapeutic targets and testing candidates for the ability to improve muscle function require cell-based model systems that can reliably predict in vivo effects in preclinical rodent models and human patients. Many useful rodent cell lines such as mouse C2C12 or rat L6 myoblasts are available. These cell lines, and others, have been extensively used to explore the molecular mechanisms of muscle differentiation and function. However, because immortalized cell lines are often genetically abnormal and maintained under artificial culture conditions for very long periods of time which can cause them to deviate from normal function, the use of primary human muscle cells may be a more predictive screening strategy.
The possibility of using in vitro obtained myoblast culture as an obvious and accessible source of myogenic cells for addressing complex muscle tissue pathologies has long attracted surgeons and trans-plantologists. Human primary myoblasts (MB) cultured in vitro could serve as an important tool to study myocytes biology, metabolism and regulation of myogenic cell differentiation. Despite their unipotent features, myoblasts could serve as a valuable source for stem cell-based therapies not only for neuromuscular disorders such as muscular dystrophy or other types of myopathies, but also for treatment of other types of muscle tissue damage such as myocardial infraction or sphincters injury.
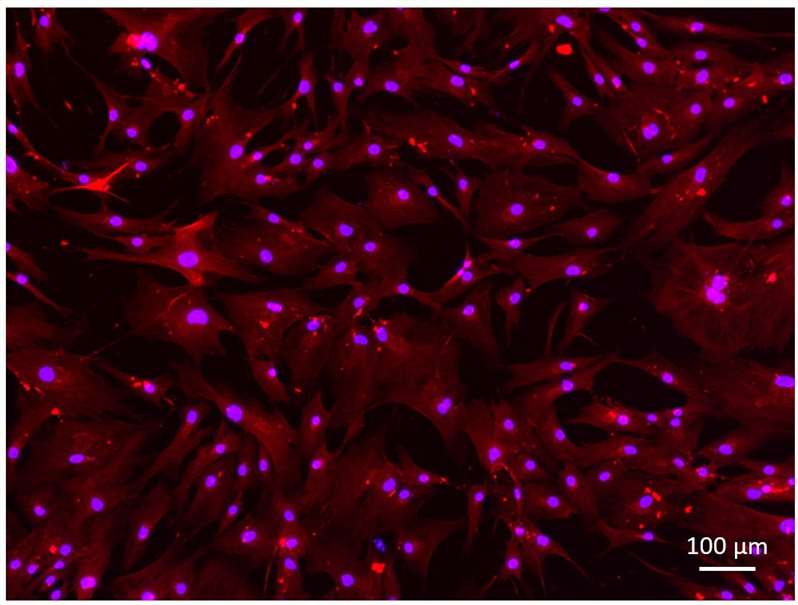 Fig. 1. Desmin staining of primary human myoblasts (Cordeiro-Santanach, Anna, et al. 2025).
Fig. 1. Desmin staining of primary human myoblasts (Cordeiro-Santanach, Anna, et al. 2025).
Expression of the CD56, CD90, CD34 and CD45 markers during myoblasts culture
The main goal of this prospective study was to evaluate the effect of myoblast isolation procedure on the stability of myoblasts phenotype and proliferation potential as well as to assess the effect of different culture media on the reproducibility and stability of the CD56 marker during myoblast propagation up to 6 passages. These two features are crucial for propagation of myoblasts with top quality and quantity and would serve as the predictive values for optimization of the myoblast culture for future development of the myoblast-based cellular therapies.
Standard myoblast isolation and in vitro culture was performed on n=27 muscle tissue biopsies. The isolated myoblasts presented expression of the myogenic markers such as desmin and after differentiation formed multinucleated myotubes which were positive for myosin heavy chain and dystrophin proteins. The typical panel of myoblasts characteristics is shown on Fig. 1. The average fusion index calculated for the cultured in vitro myoblasts reached 53% as shown in Fig. 2.
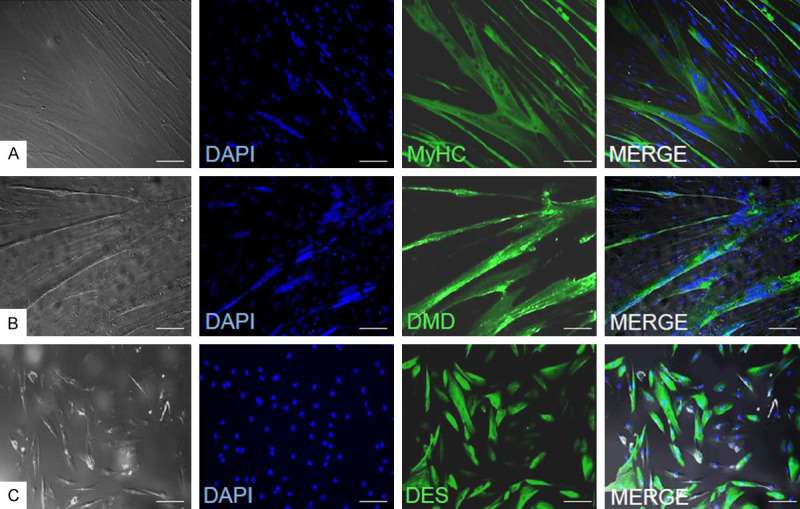 Fig. 1. Immunofluorescence assessment of myogenic markers expression in myoblasts (Rozwadowska, Natalia, et al. 2022). Myosin heavy chain (A), dystrophin (B) and desmin (C).
Fig. 1. Immunofluorescence assessment of myogenic markers expression in myoblasts (Rozwadowska, Natalia, et al. 2022). Myosin heavy chain (A), dystrophin (B) and desmin (C).
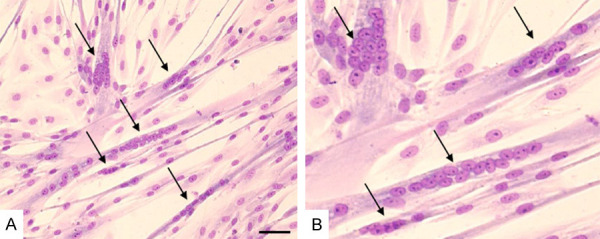 Fig. 2. Example of myotubes formation in differentiated myoblasts cultured in DMEM+FBS+FGF medium (Rozwadowska, Natalia, et al. 2022).
Fig. 2. Example of myotubes formation in differentiated myoblasts cultured in DMEM+FBS+FGF medium (Rozwadowska, Natalia, et al. 2022).
Generation of Monoculture Myogenic Sheroids
The aim of this study was to generate monoculture spheroids consisting of human skeletal myoblasts in a high-throughput manner using a microchip environment. Prior to the use of human skeletal myoblasts for the generation of myogenic spheroids, cells were characterized.
Subsequently, monoculture spheroids consisting of human skeletal myoblasts were generated using microchips. Spheroids started to disintegrate, resulting in irregular shaped spheroids after 7 and 14 d of culture. To enhance spheroid quality, ascorbic acid was added to the skeletal myoblast fusion medium (SkFM) in either a low (125 μM) or high (250 μM) concentration. Less spheroid disintegration and cell debris was observed when increasing the concentration of ascorbic acid. Live/dead analysis with calcein-AM/propidium iodide (green/red) showed the occurrence of slightly more cell death in the condition without ascorbic acid (Fig.3).
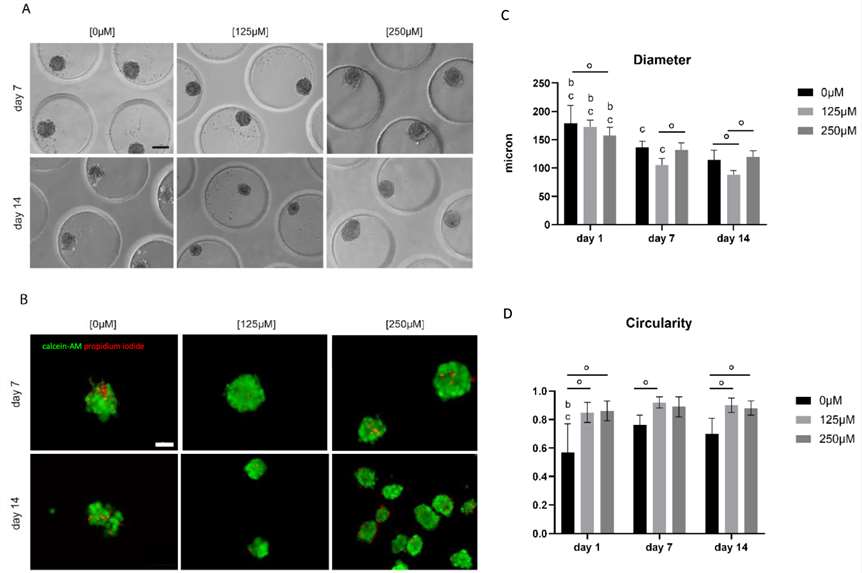 Fig. 3. Analysis of morphology, viability and morphometry of monoculture myoblast spheroids (Minne, Mendy, et al. 2024).
Fig. 3. Analysis of morphology, viability and morphometry of monoculture myoblast spheroids (Minne, Mendy, et al. 2024).
Ask a Question
Write your own review

