ONLINE INQUIRY

Human Retinal Microvascular Endothelial Cells
Cat.No.: CSC-C4361X
Species: Human
Source: Retina; Eye
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The retina serves as an essential eye component which transforms light signals into nerve impulses. Retinal microvascular endothelial cells form a critical part of the retinal microvascular system which helps sustain proper metabolism and nutrient delivery while regulating retinal homeostasis. These cells demonstrate typical endothelial cell features which encompass both a flattened shape and the production of endothelial markers including CD31 (PECAM-1) and VE-cadherin. These cells contain tight junction proteins including ZO-1, occludin, and claudin which support barrier integrity.
These cells function as pivotal models for researching vascular diseases in the retina which include diabetic retinopathy alongside retinal vein occlusion and age-related macular degeneration. The in vitro culture of human retinal microvascular endothelial cells enables researchers to mimic disease states like high glucose levels with hypoxia and inflammation to investigate disease pathogenesis. The powerful angiogenic qualities of these cells enable researchers to explore the molecular mechanisms and signaling pathways that control angiogenesis.
SGLT-2 Was Expressed on HRMECs and Dapagliflozin Mitigated High Glucose–Induced ROS and Apoptosis in Cultured HRMECs
Diabetes retinopathy (DR) is one of the most common causes of blindness due to endothelial cell death caused by elevated glucose. Sodium glucose cotransporter-2 (SGLT-2) inhibitors such as dapagliflozin (DAPA) are thought to slow DR progression, but the exact effects are unknown.
Hu et al. investigated DAPA's effects by measuring apoptotic markers in human retinal microvascular endothelial cells (HRMECs) using metabolomics, western blot and staining. It seeks to understand the role of DAPA in apoptosis and HRMEC metabolism. HRMECs were exposed to glucose concentrations (5.5, 30, 60 mmol/L) for 24 hours. WB and qPCR detected SGLT-2 in HRMECs, which was expressed more at higher glucose levels (Fig. 1A–C). With glucose at 30 mmol/L and 60 mmol/L, SGLT-2 protein levels increased by 1.66 and 2.18 times and mRNA levels by 4.68 and 13.56 times, respectively, versus 5.5 mmol/L. To determine DAPA's ROS and apoptosis impacts, DCFH-DA measured ROS, and WB quantified BAX, Bcl-2, and cleaved-caspase-3 expression. DAPA also eliminated high glucose ROS levels (Fig. 1D and G). Overdosed glucose spiked ROS levels by 23.73-fold but DAPA reduced this to 12.83-fold. High glucose and apoptotic-associated proteins were also altered by DAPA (Fig. 1E, F, H, I–L).
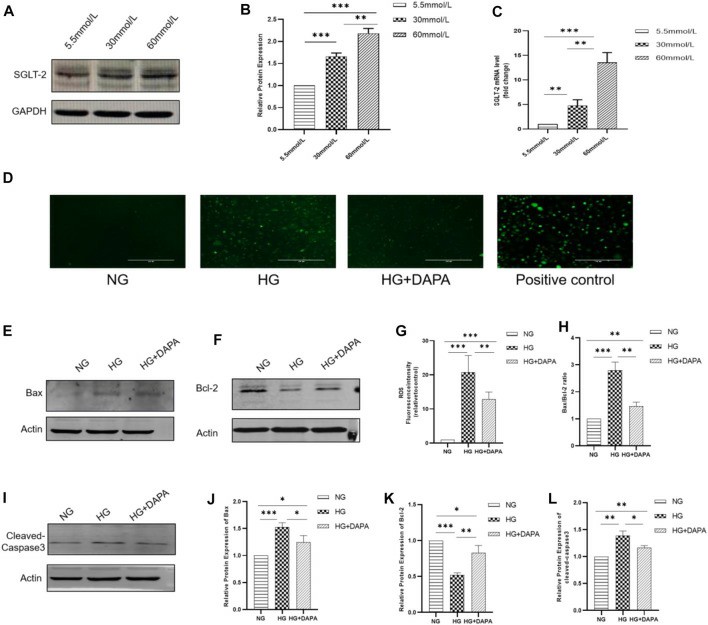 Fig. 1. Expression of SGLT-2 in HRMECs and effect of DAPA on ROS and apoptosis of HRMECs (Hu Y, Xu Q, et al., 2022).
Fig. 1. Expression of SGLT-2 in HRMECs and effect of DAPA on ROS and apoptosis of HRMECs (Hu Y, Xu Q, et al., 2022).
R59949 Reverses Hyperglycemia-Induced Cell Death and Regulates PHD2 Expression in hRMECs
Diabetes macular edema (DME) often leads to irreversible blindness and is associated with BRB breakdown due to VEGF-induced inflammation during the high-glucose state. Li et al. investigates Prolyl-4-hydroxylase 2 (PHD2) and its regulation of VEGF signaling.
In vitro, primary human retinal microvascular endothelial cells (hRMECs) were grown at hyperglycemia. As viewed by cell viability assay, hyperglycemia inhibited hRMECs' viability under glucose and mannitol conditions (Fig. 2). R59949 promoted cell death when the concentration was 5 mol/l, and DMOG suppressed it at concentrations of >400 mol/l. 5 mol/l R59949 and 300 mol/l DMOG were therefore selected for further experiments. PHD2 mRNA and protein levels were measured using RT-qPCR and western blot in order to identify whether hyperglycemia is regulating PHD2. PHD2 protein decreased 24 hours, and increased 48 hours in high-glucose conditions; mannitol behaved the same way (Fig. 3a and c). PHD2 mRNA was far higher than controls both times (Fig. 3e). R59949 was upregulated by DMOG in the presence of high glucose, while PHD2 protein and mRNA levels were downregulated (Fig. 3b, d and e).
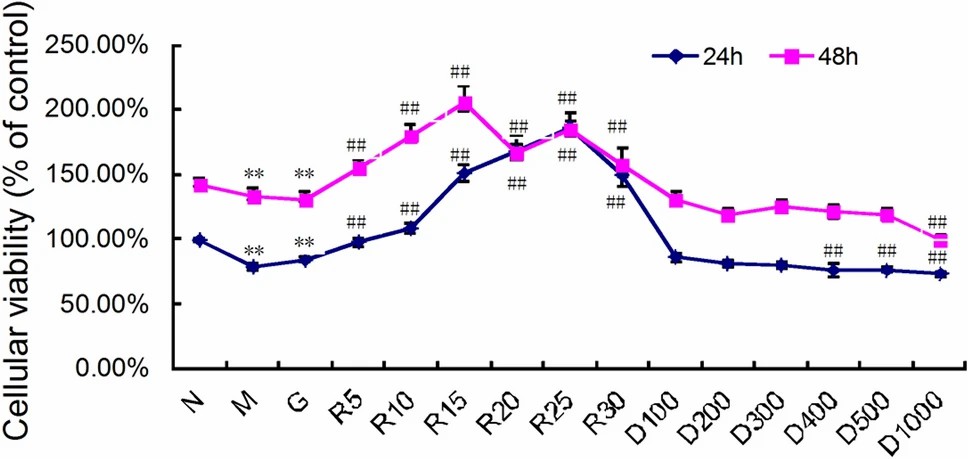 Fig. 2. The PHD2 agonist R59949 reverses the hyperglycemia-induced reduction in the viability of hRMECs (Li J, Wei L, et al., 2022).
Fig. 2. The PHD2 agonist R59949 reverses the hyperglycemia-induced reduction in the viability of hRMECs (Li J, Wei L, et al., 2022).
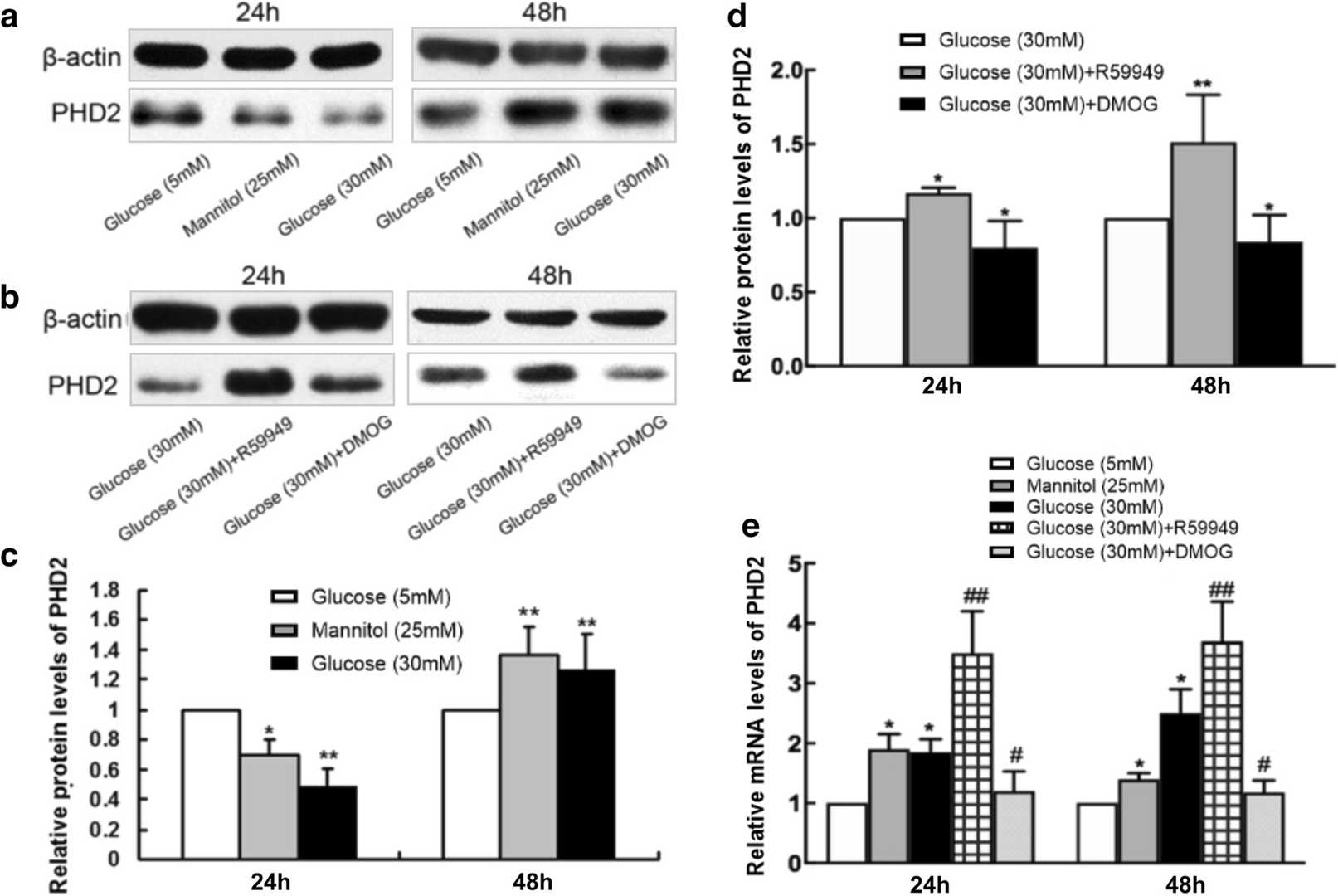 Fig. 3. Hyperglycemia alters the mRNA and protein expression of PHD2 in hRMECs (Li J, Wei L, et al., 2022).
Fig. 3. Hyperglycemia alters the mRNA and protein expression of PHD2 in hRMECs (Li J, Wei L, et al., 2022).
Ask a Question
Write your own review

