ONLINE INQUIRY

Human Ocular Choroid Fibroblasts (HOCF)
Cat.No.: CSC-7748W
Species: Human
Source: Choroid; Eye
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human ocular choroid fibroblasts (HOCF) originate from the choroid in the human eye which serves as a vital structure at the eye's back composed mainly of blood vessels and connective tissue. The choroid delivers essential nutrients and oxygen to the retina while maintaining the proper intraocular pressure within the eye. The shape of HOCF cells demonstrates typical fibroblast characteristics showing flat, polygonal, or elongated spindle formations. HOCF cells function as the main source of intraocular FABP4 (fatty acid-binding protein 4) which regulates cellular homeostasis and controls mitochondrial function alongside glycolysis. HOCF cells produce collagen and fibronectin which maintain the structural stability of the choroid. These cells participate in multiple signaling pathways including those of G protein-coupled receptors, cAMP, members of the S100 protein family, as well as adrenergic receptors.
The close relationship between HOCF cells and choroid-related diseases like choroidal neovascularization and diabetic retinopathy makes these cells vital for research into disease mechanisms and treatment approaches. These models also enable researchers to screen drugs and assess the effects of FABP4 inhibitors on cellular functions.
Effects of FABP4 on Cellular Metabolic Functions in HOCF Cells
FABP4 is present in vitreous fluid and correlates with increased fatty acid levels, suggesting it may be a key factor in RVD pathogenesis. However, the source and functions of intraocular FABP4 remain unclear. Ohruro et al. analyzed FABP4 in non-pigmented ciliary epithelium, retinoblastoma, adult retinal pigment epithelial cells, and ocular choroidal fibroblasts (HOCF) using qPCR and RNA sequencing, with the FABP4 inhibitor BMS309403 to observe metabolic impacts through Seahorse bioanalyzer.
qPCR results suggested that HOCF cells are at least one of the primary origins of FABP4 production in cells constituting the intraocular tissues. BMS309403, a selective inhibitor of FABP4, targets the fatty acid-binding pocket. Its effects on HOCF cells were examined using a Seahorse XFe96 Bioanalyzer. BMS309403 did not affect FABP4 expression in normoxia or hypoxia, confirming its protein-level interaction. Treatment with BMS309403 reduced mitochondrial respiratory functions significantly (Fig. 1A and B), while increasing basal glycolysis as indicated by ECAR, but without enhancing glycolytic reserve (Fig. 1C and D). The energy map indicated a metabolic shift from mitochondrial respiration to glycolysis in the presence of BMS309403 (Fig. 1E). Additionally, BMS309403 or hypoxia upregulated HIF1a and HIF2a mRNA expressions significantly (Fig. 2). These findings suggest that FABP4 inhibition in HOCF cells shifts metabolism and may be crucial for cellular homeostasis.
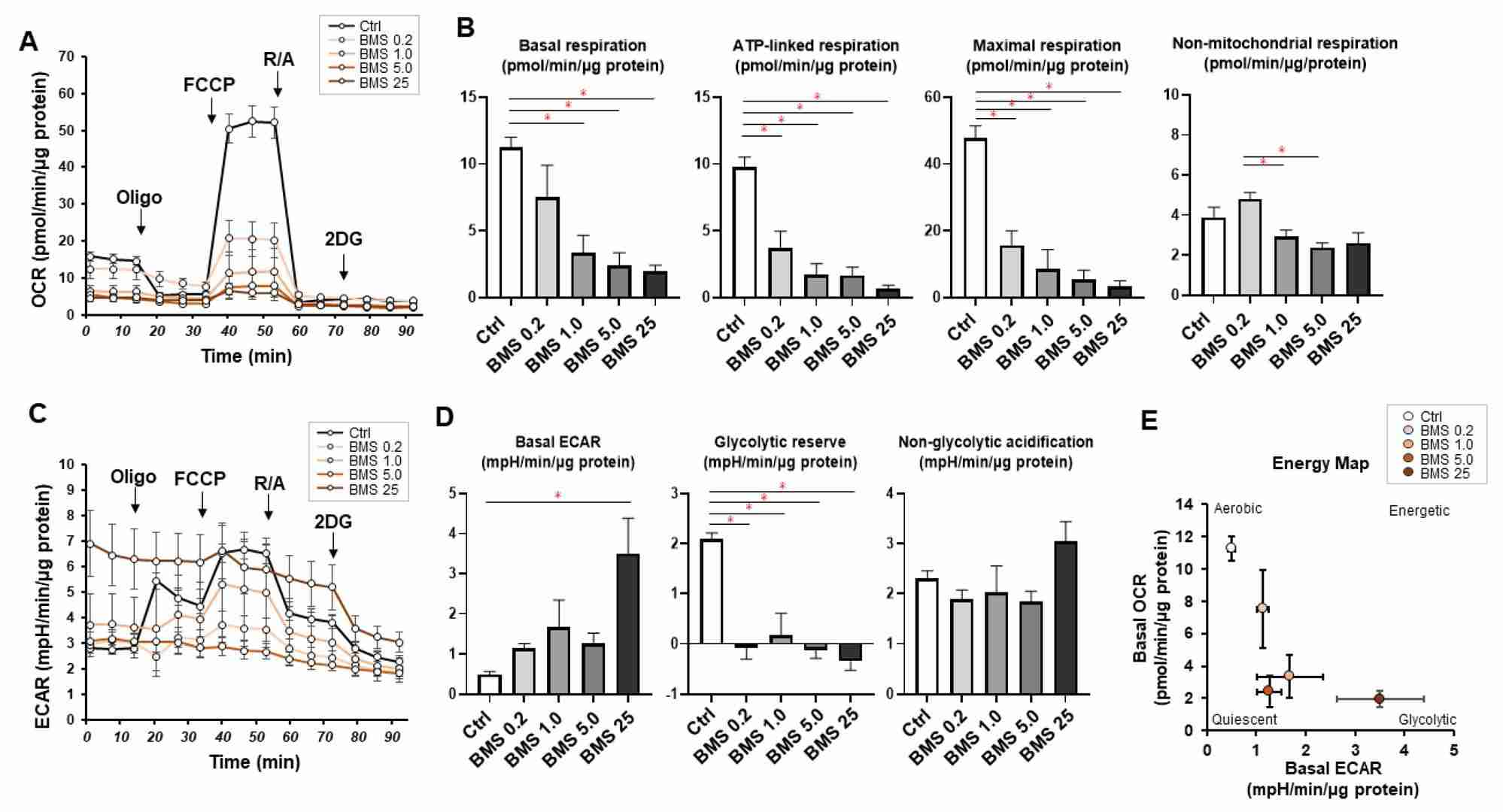 Fig. 1. Effects of pharmacological FABP4 inhibition by BMS309403 on cellular metabolic functions (Ohguro H, Watanabe M, et al., 2024).
Fig. 1. Effects of pharmacological FABP4 inhibition by BMS309403 on cellular metabolic functions (Ohguro H, Watanabe M, et al., 2024).
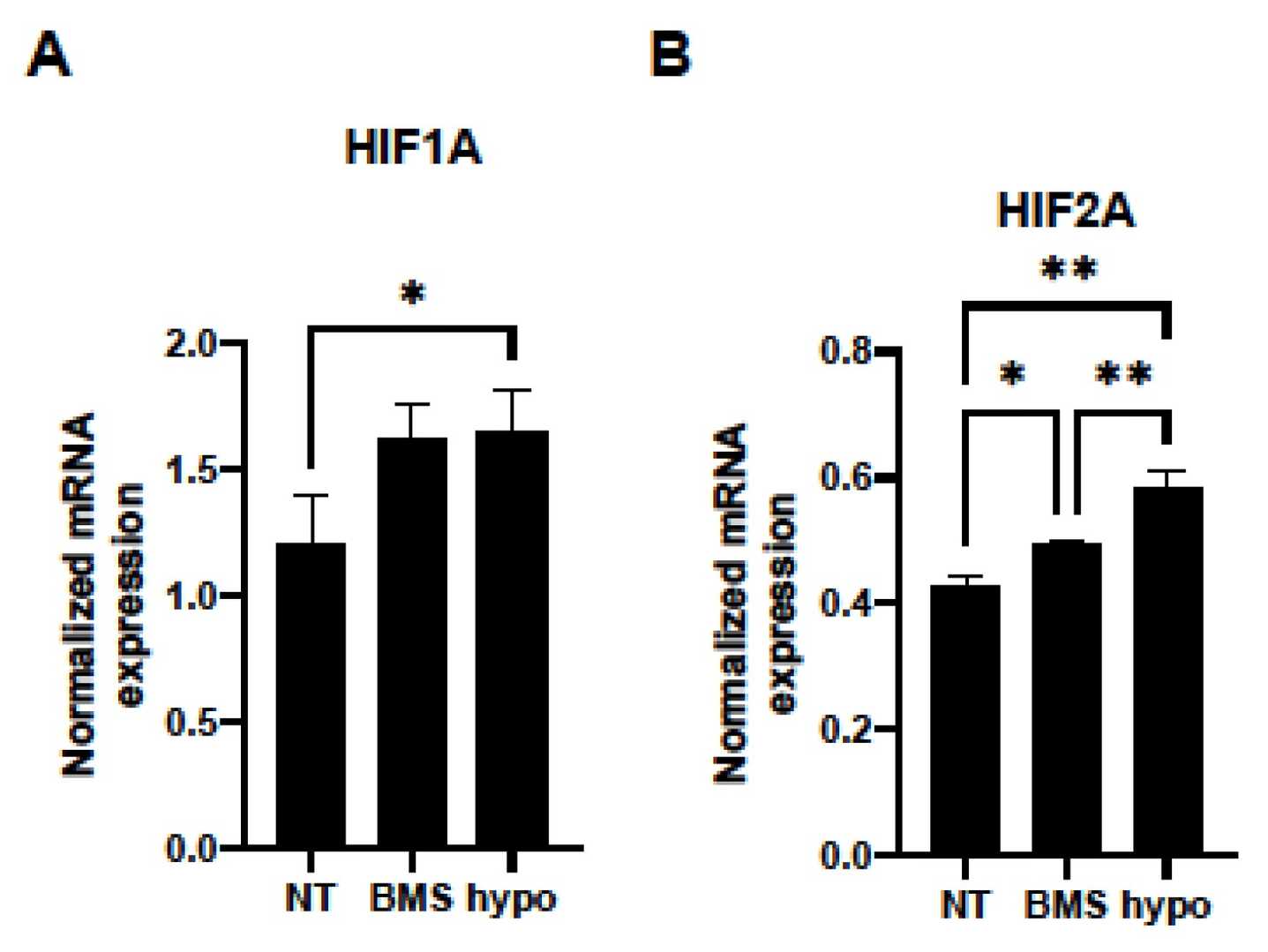 Fig. 2. mRNA expressions of (A) HIF1a and (B) HIF2a (Ohguro H, Watanabe M, et al., 2024).
Fig. 2. mRNA expressions of (A) HIF1a and (B) HIF2a (Ohguro H, Watanabe M, et al., 2024).
Atropine Differentially Modulates ECM Production by Ocular Fibroblasts
Myopia involves eye elongation due to ECM changes, with scleral thinning and choroidal alterations. Atropine slows myopia progression but its molecular action is unclear. It affects muscarinic receptors, modifying scleral and choroidal ECM. Cristaldi et al. investigated the role of human scleral fibroblast (HSF) and choroidal fibroblasts (HCOF) in myopia progression and atropine's dual effect.
Based on the hypothesis that eye elongation in myopia involves ECM protein synthesis in the sclera, and that atropine may affect this process, HSF cells were treated with atropine (0.1 to 1.0 mM) for 24 and 48 hours. Results showed dose-dependent increases in collagen type 1-α-1 and fibronectin synthesis, with collagen showing a more pronounced increase. Similarly, 7MX increased collagen and fibronectin synthesis, with fibronectin showing significant elevation only at 0.5 mM. Given the choroid's involvement in myopia and its adjacency to the sclera and retina, we analyzed the effects of atropine and 7MX on HCOF. Using 0.1 mM of each drug for 48 hours, Figure 3 illustrates that, contrary to HSF cells, both atropine and 7MX significantly reduced collagen synthesis by about 60% and fibronectin by about 80% in HCOF. This indicates atropine and 7MX might affect myopia through distinct mechanisms depending on the tissue.
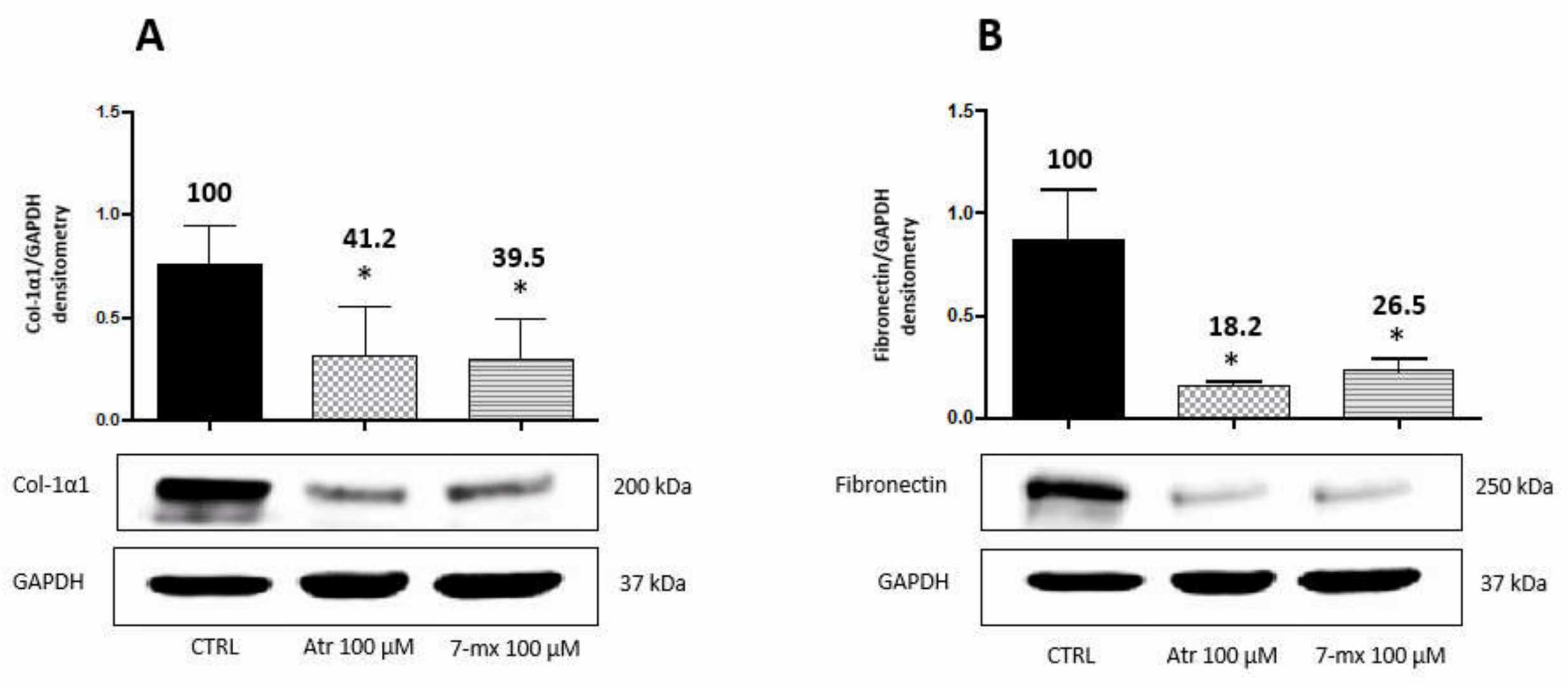 Fig. 3. Atropine and 7MX effects on collagen and fibronectin production by HCOF cells (Cristaldi M, Olivieri M, et al., 2020).
Fig. 3. Atropine and 7MX effects on collagen and fibronectin production by HCOF cells (Cristaldi M, Olivieri M, et al., 2020).
Ask a Question
Write your own review

