ONLINE INQUIRY

Human Preadipocytes-subcutaneous
Cat.No.: CSC-7736W
Species: Human
Source: Adipose
Cell Type: Preadipocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Subcutaneous adipose tissue represents body fat situated between the skin and muscle layers and mainly consists of adipocytes while containing minor amounts of connective tissue and blood vessels and nerves. This tissue performs several essential functions in the human body which include temperature regulation while also protecting internal organs and acting as an energy reserve.
Human preadipocytes-subcutaneous originate from adult subcutaneous adipose tissue. These cells demonstrate polygonal or spindle-shaped morphology while possessing intercellular connections coupled with adhesiveness. When cultured in vitro preadipocytes demonstrate surface adherence and grow to establish either single or multiple layer cell colonies. Some preadipocytes start to mature into adipocytes over time by accumulating lipid droplets and acting as a reserve of adipocytes. Furthermore, preadipocytes have the capability to produce different bioactive compounds like cytokines and hormones which help in maintaining metabolic and immune system functions throughout the body. These factors show a strong link to the development and advancement of diseases like obesity, diabetes and cardiovascular conditions. Therefore, researchers use this cell line to study both adipocyte differentiation mechanisms and lipid metabolism together with adipose tissue's ecological features. And this cell line functions as a screening platform for potential drugs targeting obesity, diabetes, and cardiovascular conditions.
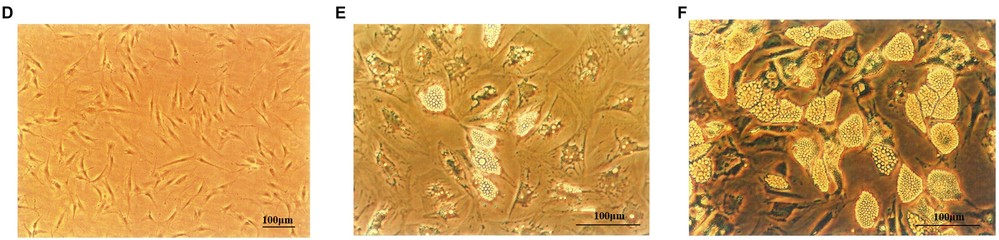 Fig. 1. Human primary preadipocytes proliferation and differentiation. (D) Human primary preadipocyte on Day 8 (×100). Adipocytes on Day 10 (E) and Day 18 (F) after inducing differentiation (×200) (Miao H, Pan H, et al., 2019).
Fig. 1. Human primary preadipocytes proliferation and differentiation. (D) Human primary preadipocyte on Day 8 (×100). Adipocytes on Day 10 (E) and Day 18 (F) after inducing differentiation (×200) (Miao H, Pan H, et al., 2019).
Hypoxanthine Secretion from Human Adipocytes
Hyperuricemia, associated with obesity and metabolic disorders, involves a delicate balance in purine metabolism. Recent murine studies reveal that adipose tissue contributes to uric acid production. Nagao et al. investigated human adipocytes and human adipose tissue by culturing freshly isolated hWAT and mWAT and measuring secreted metabolite levels. Analyzing gene expressions related to purine metabolism and metabolites under hypoxia in adipocytes further refines this understanding.
They assessed purine metabolite levels in cultured media from differentiated adipocytes via HPLC-UV. Human preadipocytes-subcutaneous were differentiated as per the manufacturer's protocol. Oil red O staining shows human preadipocytes (day 0) and differentiated adipocytes (day 8) in Supporting Information Figure 2A. Uric acid and xanthine were undetectable, but hypoxanthine was present and increased during differentiation (Fig. 1A, Fig. 2B). Given that obese adipose tissues are often hypoxic, we explored hypoxia's effect on human adipocytes. Under 1% O2 for 24 hours, hypoxanthine levels significantly rose compared to normoxia (Fig. 1A), suggesting enhanced de novo purine synthesis under hypoxia. In murine 3T3-L1 adipocytes, uric acid, not hypoxanthine or xanthine, increased in hypoxia (Fig. 1B), a pattern observed in primary murine adipocytes from subcutaneous WAT (Fig. 2C).
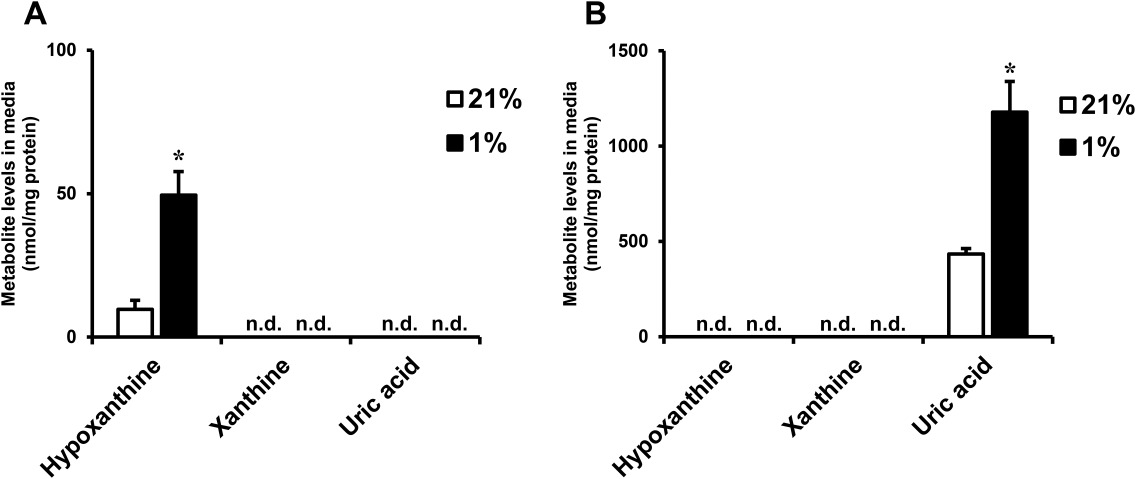 Fig. 1. Hypoxanthine levels in culture media from human adipocytes (Nagao H, Nishizawa H, et al., 2018).
Fig. 1. Hypoxanthine levels in culture media from human adipocytes (Nagao H, Nishizawa H, et al., 2018).
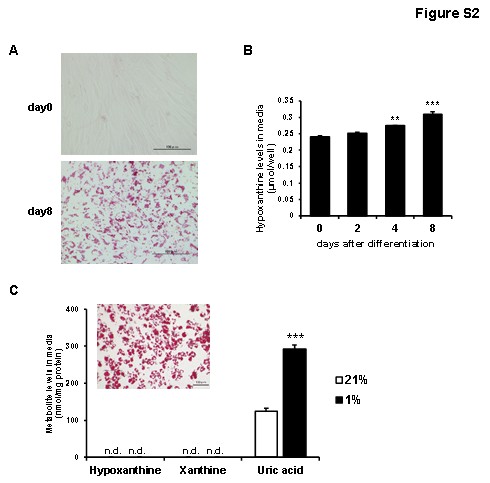 Fig. 2. Hypoxanthine levels in culture media from human preadipocytes and differentiated adipocytes (Nagao H, Nishizawa H, et al., 2018).
Fig. 2. Hypoxanthine levels in culture media from human preadipocytes and differentiated adipocytes (Nagao H, Nishizawa H, et al., 2018).
Ghrelin and IGF-1 Stimulated the Proliferation of Mouse 3T3-L1 Preadipocytes and Human Primary Preadipocytes
Obesity is tied to adipocyte proliferation influenced by transcription factors and hormones. Ghrelin, a known appetite regulator, affects adipocyte function, yet its mechanisms are not fully understood. Miao's team investigated ghrelin's role in 3T3-L1 and human preadipocyte proliferation and differentiation using MTT assay, Oil Red O staining, RT-PCR, and glycerol-3-phosphate dehydrogenase assay.
3T3-L1 preadipocytes were treated with ghrelin (0.01–1000 ng/ml) for 24 hours. Figure 3A shows that 0.01 ng/ml ghrelin significantly enhanced cell growth (107.7 ± 4.2 vs. 100.0 ± 5.9, p < 0.05), with maximal effect at 10 ng/ml (114.9% of control, p < 0.05). Higher concentrations (100 and 1000 ng/ml) slightly reduced the effect, but it remained significant (p < 0.05). Similarly, ghrelin increased proliferation in human primary preadipocytes, as shown in Figure 3C. Only 1000 ng/ml significantly boosted growth (107.8 ± 2.8 vs. 100.0 ± 3.6, p < 0.05), with no effect at lower doses (0.1 and 10 ng/ml). Figure 3D illustrates the OD value increase over 72 hours with 1000 ng/ml ghrelin, peaking at 48 hours (204.1 ± 6.1 vs. 192.1 ± 4.2, p < 0.05), disappearing by 72 hours.
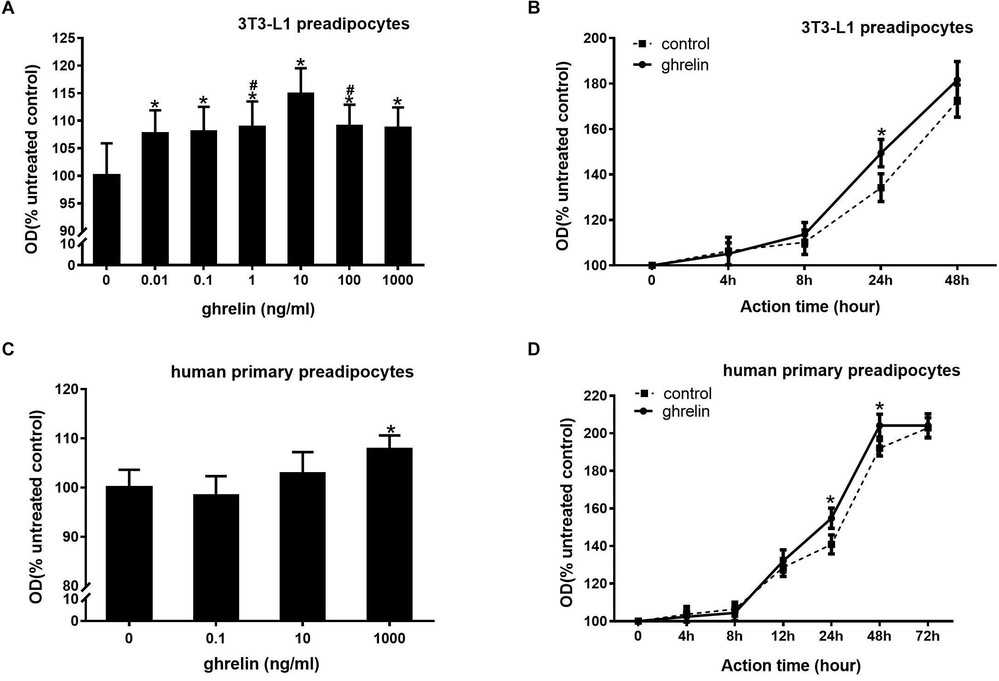 Fig. 3. Ghrelin and IGF-1 stimulated the proliferation of mouse 3T3-L1 preadipocytes and human primary preadipocytes (Miao H, Pan H, et al., 2019).
Fig. 3. Ghrelin and IGF-1 stimulated the proliferation of mouse 3T3-L1 preadipocytes and human primary preadipocytes (Miao H, Pan H, et al., 2019).
The preadipocytes are isolated from human subcutaneous adipose tissue. The adipocytes are differentiated in vitro using these isolated preadipocytes.
Preadipocytes can be trypsinized and replated several times. Preadipocytes grow slower with each passage and differentiate poorly after passage 4. Cells are shipped at Passage 2-4.
You can order preadipocytes and pre-made culture media kit (cat# CM-1145X) for adipocyte differentiation. The protocol for differentiating the cells can be found in our manual.
Yes, we often have cells from various depots available. Please inquire as to price and availability.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Easy recovering
During our experiments, the cell product was easily recovered.
19 May 2022
Ease of use
After sales services
Value for money
Write your own review