ONLINE INQUIRY

Human Intra-Hepatic Biliary Epithelial Cells
Cat.No.: CSC-C9352W
Species: Human
Source: Bile Duct
Morphology: Epithelial-like
Cell Type: Epithelial Cell; Cholangiocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human intra-hepatic biliary epithelial cells (HiBECs) are the main cell type that makes up the intrahepatic biliary tree, clustered in a tightly adherent monolayer at the surface of bile duct walls to form a 3D ductal system inside the liver. HiBECs constitute 3-5% of the total liver cell population, and they secrete and transport bile, which is released to the duodenum through the bile ducts to assist in the breakdown of fats and nutrients. HiBECs are functionally differentiated at the apical and basal membranes; the microvilli that protrude from the apical membrane vastly expand the cell surface area for efficient exchange of substances. They also have primary cilia that are sensitive to mechanical, chemical and osmotic variations, essential to the homeostasis of biliary epithelial cells.
In addition, HiBECs participate in liver metabolism and immune control by secreting chemokines and cytokines (such as TGF-β, IL-6, TNF-α) which activate and recruit immune cells to help support the body's immune response to invaders. But injury or malfunction of HiBECs can cause biliary secretion disorders with bile duct obstruction and liver disease. If liver cells are seriously damaged, HiBECs can transdifferentiate into hepatic parenchymal cells by following certain signaling routes and help regenerate liver cells. As a result, HiBECs are target cells of interest in cholangitis, cholangiocarcinoma and primary sclerosing cholangitis. To learn about HiBECs' function in liver regeneration could lead to novel perspectives and treatments in regenerative medicine.
 Fig. 1. Simple columnar epithelium of a liver bile duct.
Fig. 1. Simple columnar epithelium of a liver bile duct.
Apelin-APJ Induces Cholangiocyte Proliferation Via Nox4/ROS/ERK Signaling Pathway During Cholestasis
Apelin, a G-protein coupled receptor ligand for the APJ, is elevated in serum in a variety of human liver disorders. The apelin-APJ signaling pathway has been shown to play a role in organ fibrosis, but it is unclear what that role is. It has been shown to inhibit fibrosis in the kidney and myocardium, but appears to induce fibrosis in the liver. In cirrhotic livers, studies have found high serum apelin is associated with cirrhotic liver pathology and enhanced APJ expression.
Chen et al. attempted to evaluate apelin-APJ signaling for modulating ductular response and liver fibrosis during cholestasis. They discovered that Apelin controls Nox4 expression and evokes the generation of reactive oxygen species (ROS) that drives vascular smooth muscle cell proliferation via ERK. Hence, they speculated that apelin-APJ drives cholangiocyte growth through the Nox4/ROS/ERK pathway during cholestasis. These data showed increased NOX4 mRNA in human primary sclerosing cholangitis (hPSCL) cholangiocytes and BDL mice cholangiocytes, relative to human intra-hepatic biliary epithelial cells (HIBEpiCs) and WT, respectively, and decreases in ML221 treatment (Fig. 1A and B). Apelin increased NOX4 mRNA in HIBEpiCs in vitro, with ML221 pretreatment reversing this effect (Fig. 1C). ROS levels in cholangiocytes from BDL mice were higher compared to WT mice but were lowered by ML221 and apelin treatment (BDL+apelin) (Fig. 1D). In vitro, apelin upregulated ROS in HIBEpiCs, which was mitigated by ML221, DPI, or NAC pretreatment (Fig. 1E and F).
ML221 decreased elevated levels of ERK phosphorylation in BDL mouse cholangiocytes (Fig. 2A and B). Apelin-treated BDL mice exhibited lower levels of ERK phosphorylation than did BDL-untreated mice (Fig. 2C). Upon in vitro exposure, apelin promoted ERK phosphorylation in HIBEpiCs but not ML221, DPI, NAC or PD98059 (Fig. 2D). Apelin induced HIBEpiC proliferation, with ML221, DPI, NAC, or PD98059 pretreatment inhibiting this proliferation. PCNA and KI67 expression were also increased by apelin in HIBEpiCs, but reversed by pretreatment of the aforementioned inhibitors (Fig. 2F).
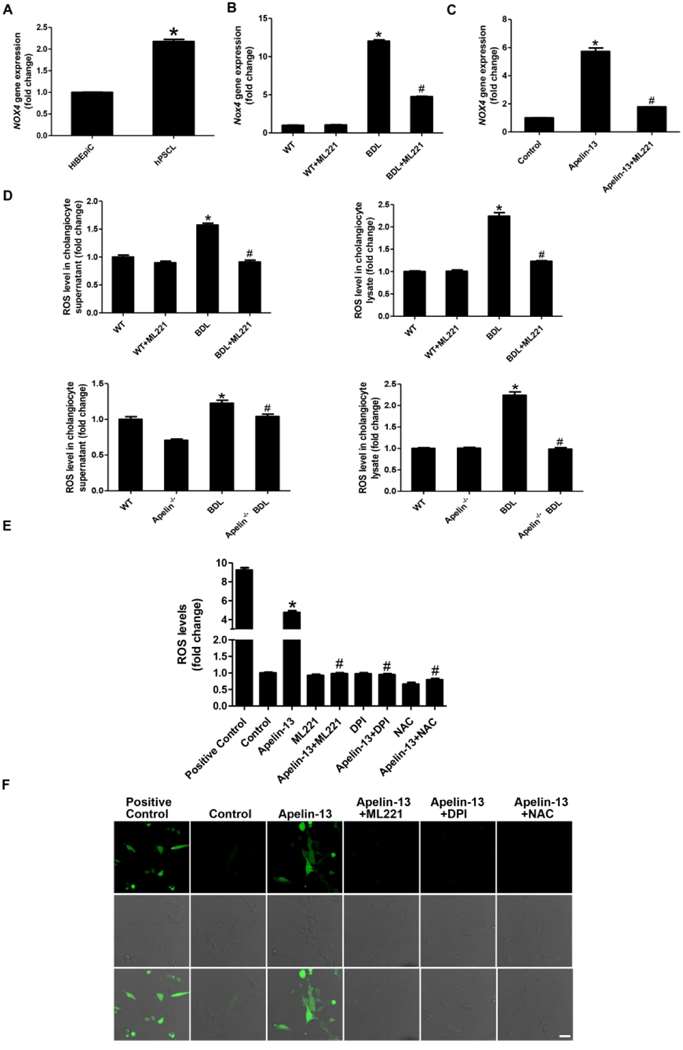 Fig. 1. Detection of Nox gene expression levels (A-C). Detection of ROS expression levels (D-E). (F) Confocal microscopy assay for intracellular ROS regeneration in HIBEpiCs (Chen L, Zhou T, et al., 2021).
Fig. 1. Detection of Nox gene expression levels (A-C). Detection of ROS expression levels (D-E). (F) Confocal microscopy assay for intracellular ROS regeneration in HIBEpiCs (Chen L, Zhou T, et al., 2021).
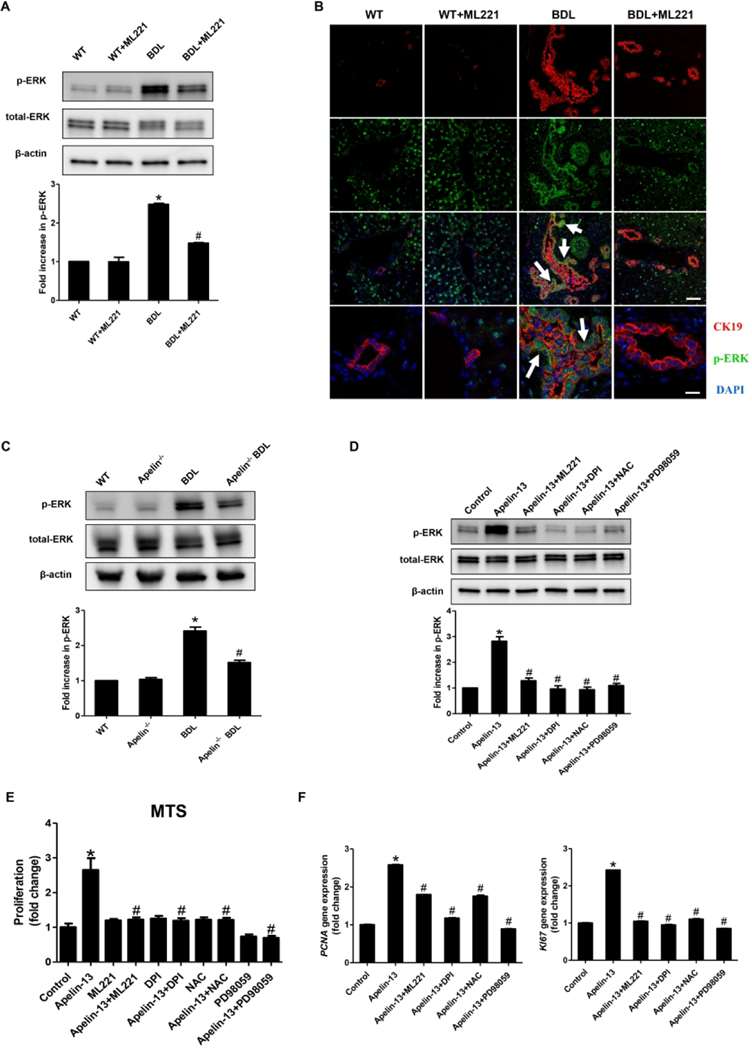 Fig. 2. The expression of p-ERK in mouse cholangiocytes, frozen liver sections and HIBEpiCs (A-D). (E) The measurement of proliferation in apelin treated HIBEpiCs. (F) The mRNA expression of PCNA and KI67 in apelin treated HIBEpiCs (Chen L, Zhou T, et al., 2021).
Fig. 2. The expression of p-ERK in mouse cholangiocytes, frozen liver sections and HIBEpiCs (A-D). (E) The measurement of proliferation in apelin treated HIBEpiCs. (F) The mRNA expression of PCNA and KI67 in apelin treated HIBEpiCs (Chen L, Zhou T, et al., 2021).
Sirt6 Ameliorated the GCDC‑Induced HiBEC Apoptosis
Cholestatic liver disorders expose biliary epithelial cells (BECs) and hepatocytes to the harmful bile acids, resulting in cell death and mitochondrial damage. It is not clear how this works on a molecular level. Studies have shown that Sirt6 can control liver damage and metabolism, but little is known about its effects on human biliary epithelial cells (HiBECs) during cholestatic disorders. Therefore, Li et al. aimed to characterize molecular basis for Sirt6 defence against HiBEC apoptosis in the presence of the bile acid glycochenodeoxycholate (GCDC).
Li et al. found that Sirt6 mitigates GCDC-induced apoptosis in human HiBECs by upregulating PGC-1's AMPK-mediated expression and deacetylation. Given that GCDC reduced Sirt6 expression in HiBECs, they then wanted to understand the impact of Sirt6 on GCDC-induced cellular damage in HiBECs. For 48 hours, HiBEC were transfected with a Sirt6 expression vector or vector control and then treated for 8 hours with 1mM GCDC. Sirt6 transfection significantly enhanced Sirt6 mRNA and protein expression (Fig. 3a), enhanced cell viability (Fig. 3b), and reduced apoptosis (Fig. 3c). Sirt6 knockdown through si-Sirt6 transfection decreased cell survival and led to greater cell death (Fig. 3d-f). The western blot analysis revealed that Sirt6 overexpression reduced the cleaved caspase-3 and Bax expression and boosted Bcl-2 levels, supporting its anti-apoptotic function. Sirt6 knockdown did exactly the opposite. Further, Sirt6 overexpression decreased cytochrome c release from mitochondria while knockdown increased it, implicating Bax, Bak and MPTP (Fig. 3g and h).
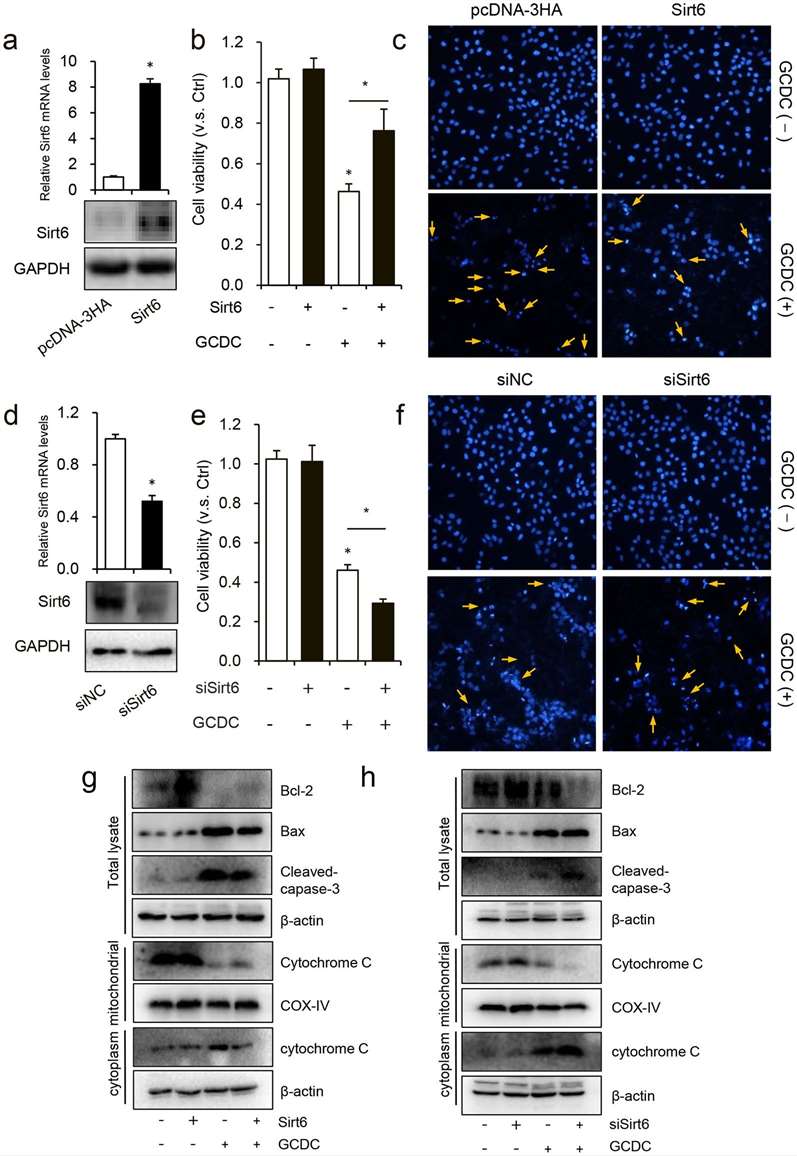 Fig. 3. Sirt6 ameliorated GCDC-induced HiBEC apoptosis (Li J, Yu D, et al., 2020).
Fig. 3. Sirt6 ameliorated GCDC-induced HiBEC apoptosis (Li J, Yu D, et al., 2020).
Ask a Question
Write your own review







