ONLINE INQUIRY

Human Gingival Epithelial Cells
Cat.No.: CSC-C9393W
Species: Human
Source: Gingiva; Periodontium
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The gingival epithelial cells (HGECs) originate from gingival tissue which serves as an essential element of the oral mucosa between teeth and the alveolar bone. This tissue comprises epithelial cells with connective tissue and blood vessels. Gingival epithelial cells perform an essential protective role for teeth and alveolar bone structures. These cells produce antimicrobial peptides like β-defensin which enable them to combat pathogens while regulating immune responses to protect the oral mucosa. Additionally, HGECs generate cytokines such as IL-8 and IL-10 to regulate immune functions and inflammatory responses. HGECs demonstrate multiple shape variations which typically manifest as polygonal or irregular forms. These cells develop layered formations similar to the stratified squamous epithelium present in human gingiva. They develop standard epithelial properties in culture environments which include strong cell-cell attachments and clear cell polarity.
In periodontal disease research, HGECs are invaluable. Researchers use them extensively to explore the pathogenesis of periodontitis, inflammatory processes, and interactions between host tissues and microbes. For example, researchers examine HGECs to determine the effects of mechanical stress on gingival tissue and to investigate how oral microbial populations impact the functioning of the gingival epithelial barrier. Furthermore, HGECs play a crucial role in developing innovative treatment approaches including cell-based techniques to restore periodontal tissue.
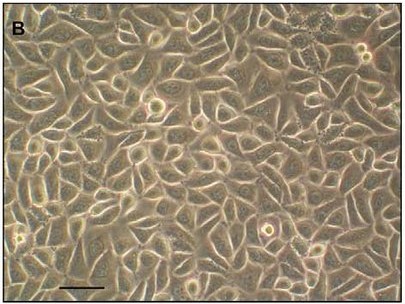 Fig. 1. Primary human gingival epithelial cells (Volponi AA, Kawasaki M, et al., 2013).
Fig. 1. Primary human gingival epithelial cells (Volponi AA, Kawasaki M, et al., 2013).
Adhesion to and Invasion of HGECs by M-PgPs
Periodontitis is a widespread, biofilm-associated inflammatory disease. The pathogen Porphyromonas gingivalis plays a crucial role in disrupting host-microbe balance, leading to disease. Wang's team explored how metronidazole-tolerant P. gingivalis persisters (M-PgPs) interact with human gingival epithelial cells, given their role in maintaining infection through persistence and resistance to treatment.
As adhesion to and invasion of host cells are two important virulence factors of P. gingivalis, they attempted to determine whether M-PgPs could maintain such critical capabilities. M-PgP- and P. gingivalis-treated HGECs were assessed with immunofluorescence (IF), TEM, and flow cytometry. Importantly, the M-PgPs were still able to adhere to the cell surfaces and yet invade the cytoplasts as P. gingivalis did (Fig. 1a), while heat-killed M-PgPs lost such pathogenic abilities (Fig. 1d and e). TEM findings demonstrated that M-PgPs altered the cell membrane and led to the generation of membrane ruffles, thereby facilitating the invasion of HGECs, whereas the membranes of HGECs remained unchanged, even when heat-killed M-PgPs were present quite near them (Fig. 1f to j). In addition, similar proportions of HGECs were adhered to or invaded by M-PgPs and P. gingivalis as quantitated by flow cytometry (Fig. 2).
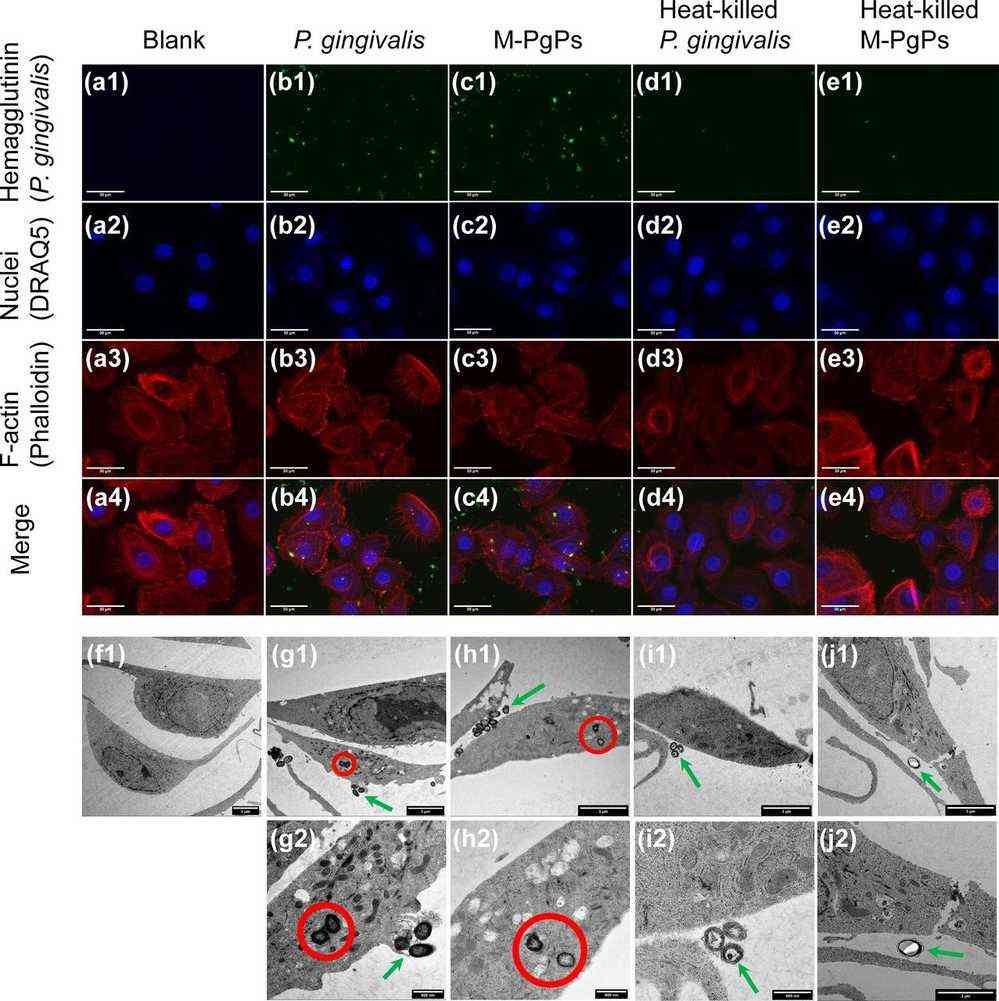 Fig. 1. Adhesion to and invasion of HGECs by M-PgPs (Wang C, Cheng T, et al., 2020).
Fig. 1. Adhesion to and invasion of HGECs by M-PgPs (Wang C, Cheng T, et al., 2020).
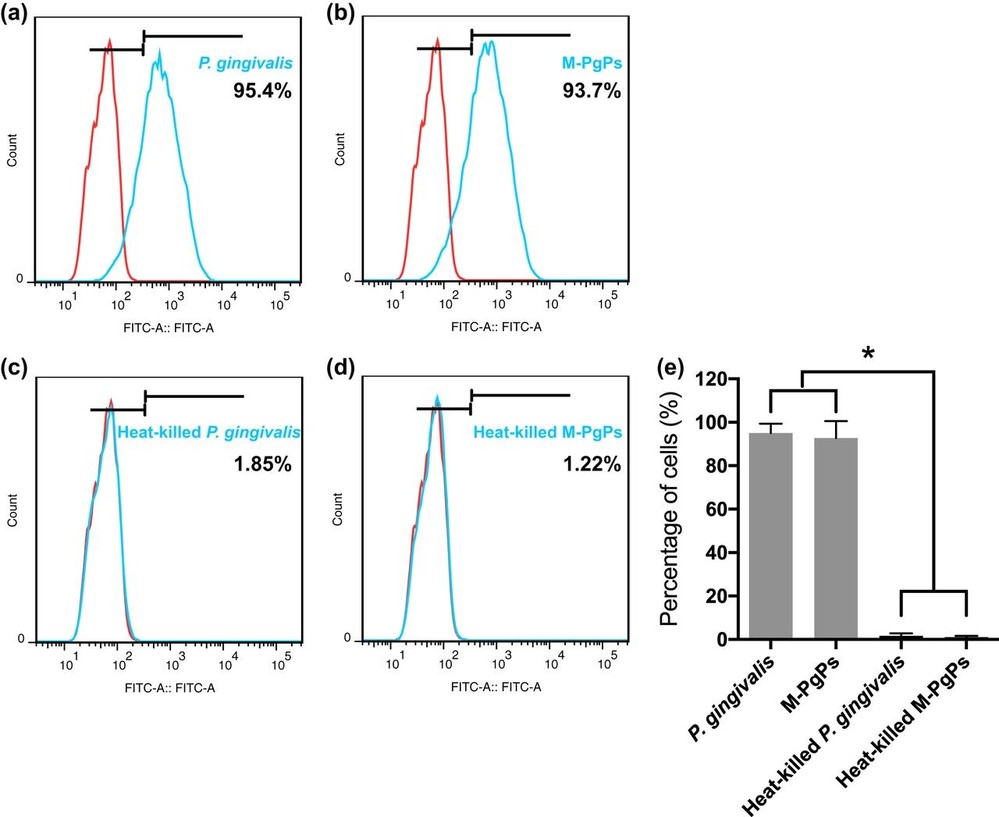 Fig. 2. Quantitation of HGECs adhered to and invaded by M-PgPs (Wang C, Cheng T, et al., 2020).
Fig. 2. Quantitation of HGECs adhered to and invaded by M-PgPs (Wang C, Cheng T, et al., 2020).
Cigarette Smoke Condensates Promote SARS-CoV-2 Pseudovirus Internalization via AhR Activation in Gingival Epithelial Cells
Smoking is a significant health risk linked to diseases like cancer and respiratory issues. Recent studies suggest it may increase susceptibility to COVID-19. Almeida-da-Silva et al. investigated how cigarette smoke affects SARS-CoV-2 receptor expression and viral infection in human gingival epithelial cells (GECs).
SARS-CoV-2 is known to infect cells in the oral cavity and saliva. ACE2, crucial for SARS-CoV-2 infection, sees increased expression in GECs after TCDD or CSC treatment. Almeida-da-Silva et al. hypothesized these treatments might enhance viral infection. GECs were pretreated with TCDD or CSC, then exposed to a GFP-tagged SARS-CoV-2 pseudovirus. Our experiments confirmed that the virus could infect both untreated and treated GECs (Fig. 3A). Particularly, 10 μg/mL CSC pretreatment significantly elevated infection levels by increasing ACE2 expression (Fig. 3B). Thus, their data suggest that CSC treatment increases the levels of ACE2 in GECs, which enhances SARS-CoV-2 pseudovirus internalization. Since CSC induces AhR activation in GECs and AhR activation increases ACE2 expression in these cells, they further investigated whether CSC amplifies infection through AhR activation. Using siRNA to target AhR before CSC exposure showed a decrease in virus internalization in GECs (Fig. 4A). GECs with reduced AhR displayed lower infection compared to control (Fig. 4B). Thus, CSC elevates ACE2 levels via AhR, fostering SARS-CoV-2 pseudovirus infection.
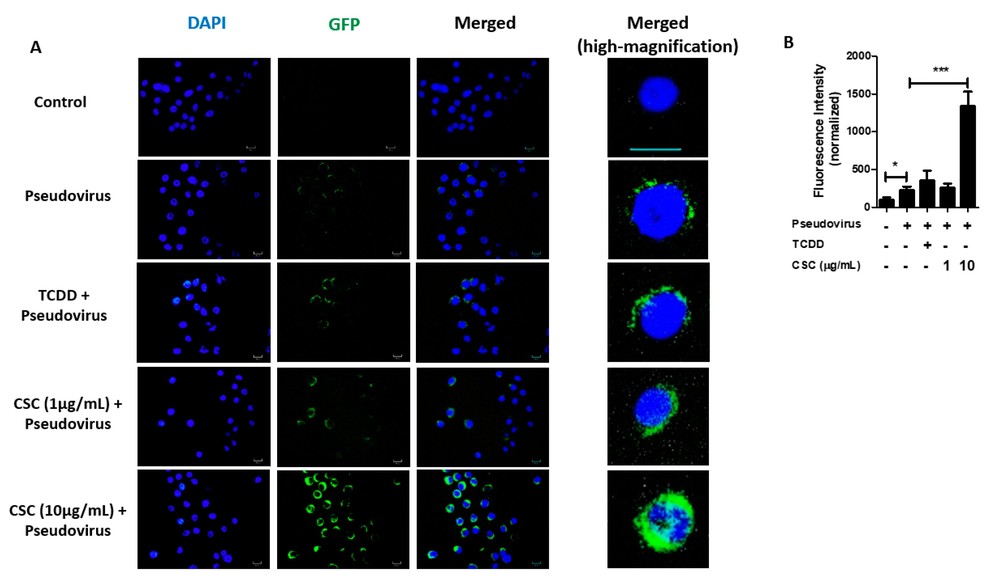 Fig. 3. Pretreatment with cigarette smoke condensates increases severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pseudovirus infection in gingival epithelial cells (Almeida-da-Silva CLC, Matshik Dakafay H, et al., 2021).
Fig. 3. Pretreatment with cigarette smoke condensates increases severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pseudovirus infection in gingival epithelial cells (Almeida-da-Silva CLC, Matshik Dakafay H, et al., 2021).
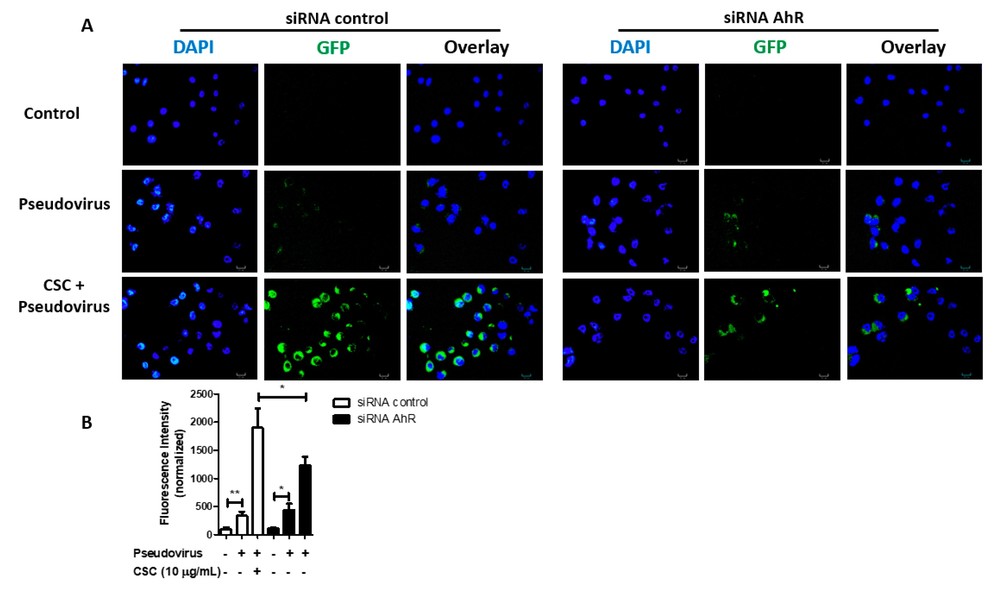 Fig. 4. Treatment with cigarette smoke condensates increases severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pseudovirus infection in gingival epithelial cells via aryl hydrocarbon receptor (AhR) signaling (Almeida-da-Silva CLC, Matshik Dakafay H, et al., 2021).
Fig. 4. Treatment with cigarette smoke condensates increases severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pseudovirus infection in gingival epithelial cells via aryl hydrocarbon receptor (AhR) signaling (Almeida-da-Silva CLC, Matshik Dakafay H, et al., 2021).
Ask a Question
Write your own review