ONLINE INQUIRY

Human Dermal Fibroblasts-neonate (HDF-n)
Cat.No.: CSC-7795W
Species: Human
Source: Dermis; Skin
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human dermal fibroblasts-neonate (HDF-n), which originate from newborn skin, are key cells in keeping neonatal skin intact and functional. Morphologically, these cells are often spindle or flattened stellates with prominent protrusions and a large, distinct nucleus. The microvilli and filopodia on their surface allow for the release and reorganisation of the ECM.
In the neonatal period, the skin is subject to major changes between prenatal and postnatal life that are marked by explosive development. By this time, HDF-n are very active, producing ECM constituents including collagen (mainly type I and III collagen), elastin, glycosaminoglycans and glycoproteins to keep tissues elastic and robust. In comparison with adult dermal fibroblasts, HDF-n is more proliferative, more metabolic, and responsive to and quicker to respond to external agents, including inflammation and injury, speeding the repair and regeneration process.
HDF-n are widely used to investigate skin issues, such as ageing, cancer and scars. They are the vital seed cells that renew and repair skin, tendons and connective tissues. Furthermore, the fibroblasts act as feeder layers for iPSCs, providing an environment for stem cell proliferation and differentiation, furthering their use in regenerative medicine and tissue engineering.
The In Vitro Effect of Analyzed Genes on the CTGF, TGFBR2, and DNMT3A Protein Levels
During skin ageing, fibroblasts are controlled by transforming growth factor β (TGF-β) signalling, which is dependent on CTGF and DNA methyltransferases (DNMT). In order to examine the possibility that the regulation of TGF-β signalling and related molecular pathways could vary at different developmental stages, Tomela's team knocked out TGFB1, TGFB3, TGFBR2, CTGF, DNMT1, and DNMT3A genes in neonatal (HDF-N) and adult (HDF-A) human dermal fibroblasts, and studied changes in CTGF, TGFBR2 and DNMT3A protein levels.
CTGF protein levels declined 68% after DNMT1 was silenced in HDF-N cells but not in HDF-A cells. There were no changes in CTGF after knockdown of TGFBR2, TGFB1, TGFB3, or DNMT3A. TGFB1 in either cell line. TGFB1 knockdown reduced TGFBR2 expression by 52% in HDF-A but not HDF-N cells. TGFB3, DNMT1, and DNMT3A silenced TGFBR2 by 62%, 67% and 37%, respectively, in HDF-N, but not in HDF-A. TGFBR2 didn't respond to CTGF knockdown, and TGFBR2 silencing inhibited DNMT3. A by 37% in HDF-N cells, with similar reductions after TGFB3 and DNMT1 knockdown. No effect of TGFB3 or DNMT1 silencing on the DNMT3A protein amount was observed in HDF-A cells. The level of DNMT3A also remained unchanged in both cell lines after the treatment with CTGF and TGFB1 siRNAs (Fig. 1).
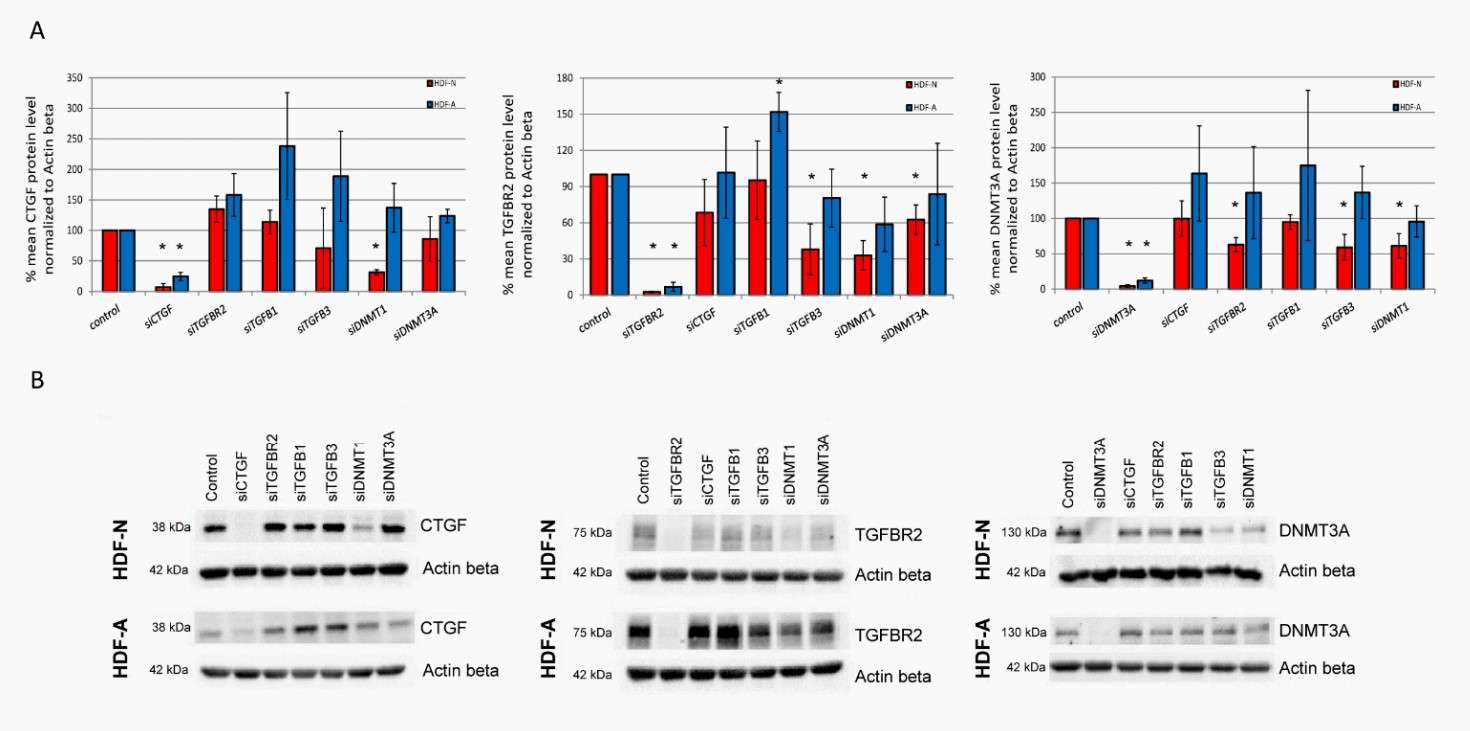 Fig. 1. The protein levels of CTGF, TGFBR2 and DNMT3A in neonatal (HDF-N) and adult (HDF-A) human dermal fibroblast cell lines (Tomela K, Karolak JA, et al., 2021).
Fig. 1. The protein levels of CTGF, TGFBR2 and DNMT3A in neonatal (HDF-N) and adult (HDF-A) human dermal fibroblast cell lines (Tomela K, Karolak JA, et al., 2021).
Small Molecules Can Efficiently Reprogram Fibroblasts to Neurons
Recent studies have shown that fibroblasts can be directly converted into functional neurons by small molecules, but the low efficiency of such chemically induced neurons (iNs) limits their application. Yang's team investigated the reprogramming ability of human fibroblasts when continuously exposed to a combination of small molecules.
They found that human newborn foreskin fibroblasts (HNFFs) can be efficiently and directly reprogrammed into glutamatergic neuron-like cells through the combination of small molecules. HNFFs began shrinking by day 4, with lamellipodia contracting and cell somas rounding by day 7, unlike controls with 1% DMSO which retained spindle shapes (Fig. 2D). Reprogramming efficiency peaked at day 14 with 82.1% ± 1.6% TUJ1-positive cells (Fig. 2D and E). Efficiency dropped by day 21, likely due to small molecule toxicity confirmed by rising apoptosis rates through caspase-3 expression. Further analysis showed iNs expressed neuronal markers like DCX, MAP2, NeuN, and TAU with high double-positive rates alongside TUJ1 (Fig. 2H, 2J–L, 2M). Control wells showed minimal marker expression (Fig. 2G and I).
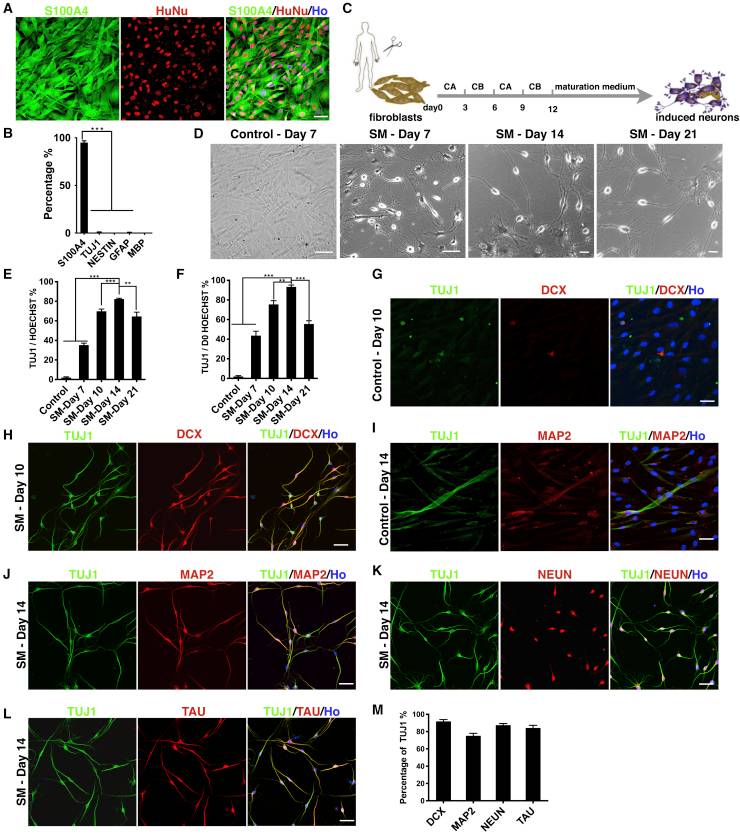 Fig. 2. Small molecules can efficiently reprogram fibroblasts to neurons (Yang Y, Chen R, et al., 2019).
Fig. 2. Small molecules can efficiently reprogram fibroblasts to neurons (Yang Y, Chen R, et al., 2019).
Ask a Question
Write your own review