ONLINE INQUIRY

Human Astrocytes-spinal cord (HA-sp)
Cat.No.: CSC-7831W
Species: Human
Source: Spinal Cord
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The Human Astrocytes-spinal cord (HA-sp) cell line consists of astrocytes derived from the human spinal cord. Morphologically, these cells are composed of a cell body and various processes, which further branch into extensions, small branches, and leaflets. These structures fill and support the spaces between neuronal cell bodies and their processes. They not only support the structure of neurons, but they also function as insulators to keep the flow of signals between neurons tidy.
These spinal cord astrocytes are diverse in structure and function. They connect neurons and blood vessels, helping to transfer nutrients and preserve the spinal cord's homeostasis. Additionally, these cells keep neurons electrolyte hydrated and replenish neurotransmitters. They assist with the repair of injuries to the central nervous system, by proliferating and morphologically changing. The HA-sp cell line is invaluable in scientific research, especially for the development of in vitro models for spinal cord disorders. By mimicking pathology – ischemia, hypoxia, inflammation or genetic mutations – researchers can probe the pathology of disorders such as spinal cord injury, myelitis and spinal muscular atrophy. With the HA-sp cell line as a model platform for in vitro drug screening, it is also possible to assess the therapeutic drug's impact on the activity of spinal cord astrocytes.
Effect of Riluzole, Curcumin, and Their Combination on H2O2-Induced Cell Death in Astrocytes
Spinal cord injury (SCI) presents a serious medical challenge, often resulting in irreversible motor and sensory impairments that do not yet have a cure. Riluzole, a sodium channel-blocker and glutamate inhibitor, is in preclinical use for SC injury (SCI), and curcumin is an intracellular calcium inhibitor that attenuates glutamate-induced neurotoxicity. Daverey et al. investigated the neuroprotective effects of a combination of riluzole and curcumin in human astrocytes and white matter injury (WMI) model of SCI.
The combination of riluzole and curcumin was tested on human astrocytes-spinal cord (HA-sp) to evaluate its effects on oxidative stress induced by H2O2. The MTT test showed substantial cell death at all H2O2 concentrations (Fig. 1A), especially at 400 M, which killed about 30% of cells and was applied to induce oxidative stress in the experiment. Riluzole was tested at 1 M, 5 M, and 10 M along with 1 M curcumin. The presence of both riluzole and curcumin significantly increased cell survival relative to the control at 1 M (Fig. 1B). Yet only at 1 M did both drugs inhibit H2O2-mediated cell death, whereas at 5 M and 10 M riluzole did not substantially inhibit cell death (Fig. 1C). Combining riluzole (1 M) and curcumin resulted in significantly less cell death than H2O2-treated astrocytes. But curcumin on its own dominated the mix, suggesting that the mix is not particularly helpful for suppressing H2O2-induced apoptosis. Notably, the combination of riluzole (10 M) and curcumin induced more cell death than H2O2 itself (Fig. 1D).
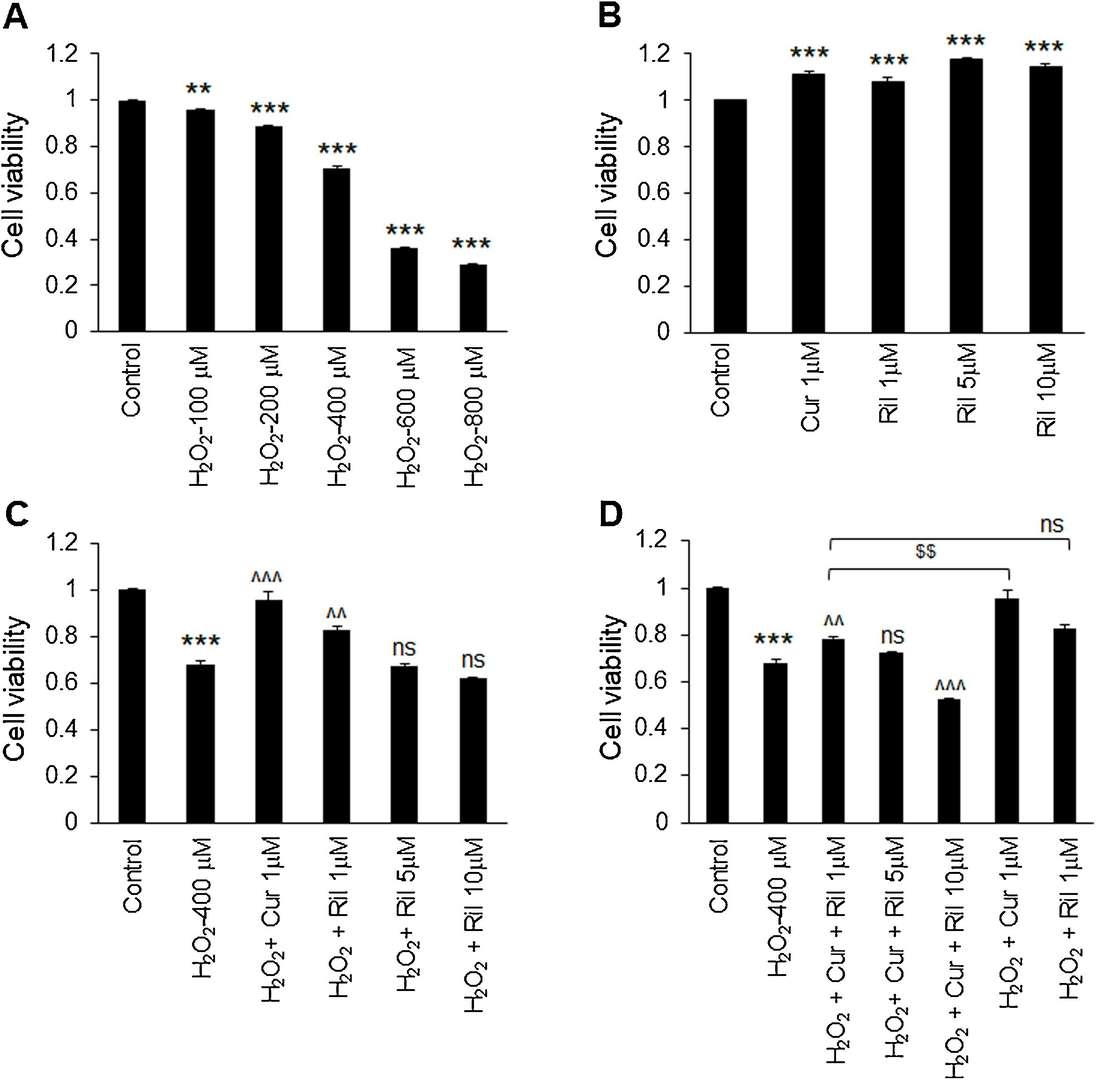 Fig. 1. Effect of riluzole and curcumin combination on H2O2-induced cell death in astrocytes (Daverey A, Agrawal SK, et al., 2020).
Fig. 1. Effect of riluzole and curcumin combination on H2O2-induced cell death in astrocytes (Daverey A, Agrawal SK, et al., 2020).
VZV Infection is Associated with Nuclear Localization of NK-1R in Primary Human Spinal Astrocytes
Varicella zoster virus (VZV) reactivates with age or immunosuppression, potentially infecting the spinal cord and causing myelopathy. Astrocytes within the spinal cord are believed to be key in VZV's central nervous system spread. Bubak et al. assessed primary human spinal astrocytes infected with VZV for substance P production, NK-1R localization, and viral spread, with and without NK-1R antagonists. To explore the role of the neurokinin-1 receptor (NK-1R) in VZV-infected human spinal astrocytes.
Three days post-infection, IFA showed NK-1R localization in cell membrane/cytoplasm of mock-infected qHA-sps (Fig. 2A), with minimal nuclear presence (Fig. 2B). Conversely, VZV-gE-positive qHA-sps had NK-1R mainly in the nucleus (Fig. 2C and 2D), confirmed by z-stack imaging. Early infection was evident in qHA-sps at the VZV-infected cluster edges, identified by slight VZV gB presence at the nucleus base (Fig. 2E) and initial NK-1R nuclear localization. Exposure to VZV lysates resulted in nuclear NK-1R in VZV-gB–positive cells, unlike UV-irradiated lysates, which showed none. After three days, flow cytometry revealed that only 20% of the cells were infected (Fig. 3A), ensuring the cultures weren't overly infected. Western blot showed a distinct lamin band (66 kDa) in nuclear fractions. Using a polyclonal anti–NK-1R antibody, three NK-1R isoforms were observed (46-kDa full-length and 38- and 33-kDa truncated isoforms) (Fig. 3B). The 38-kDa isoform predominantly appeared in the nucleus following VZV infection, while the other isoforms also shifted significantly to the nucleus. Band analysis showed a shift in full-length and 33-kDa NK-1R expressions from cytoplasm to nucleus upon VZV infection (Fig. 3C). PCR amplification of NK-1R revealed more amplified product from primers that detected all isoforms than from primers that detected only the full-length isoform, suggesting that RNA from truncated isoforms was also contributing to total amplified products. There was not a notable change in full-length or all NK-1R transcripts during VZV infection.
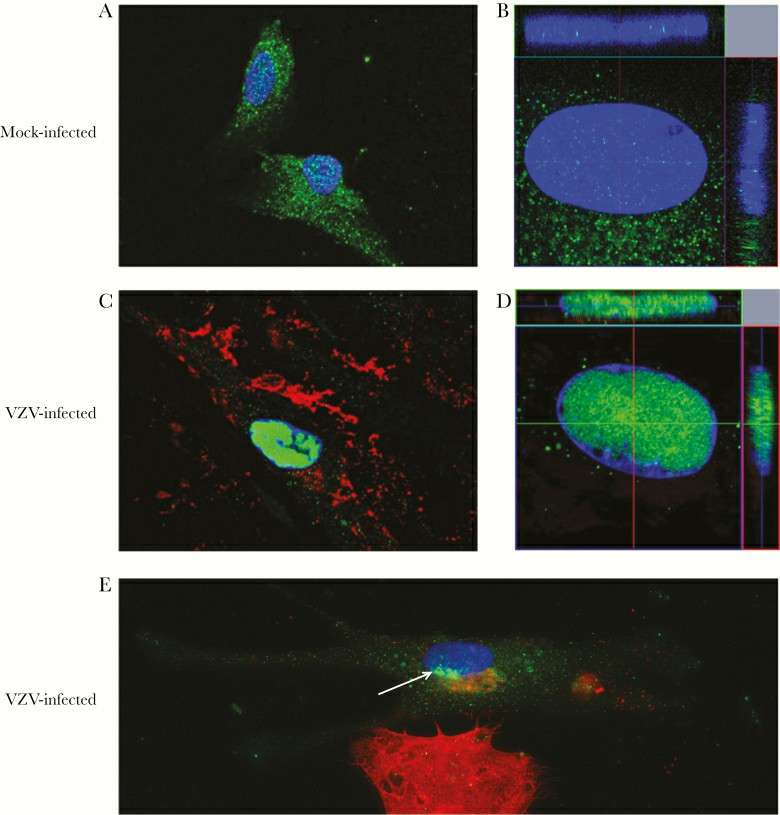 Fig. 2. Neurokinin-1 receptor localization in mock- and varicella zoster virus (VZV)–infected primary human spinal astrocytes, detected by immunofluorescence antibody assay (IFA) (Bubak AN, Como CN, et al., 2018).
Fig. 2. Neurokinin-1 receptor localization in mock- and varicella zoster virus (VZV)–infected primary human spinal astrocytes, detected by immunofluorescence antibody assay (IFA) (Bubak AN, Como CN, et al., 2018).
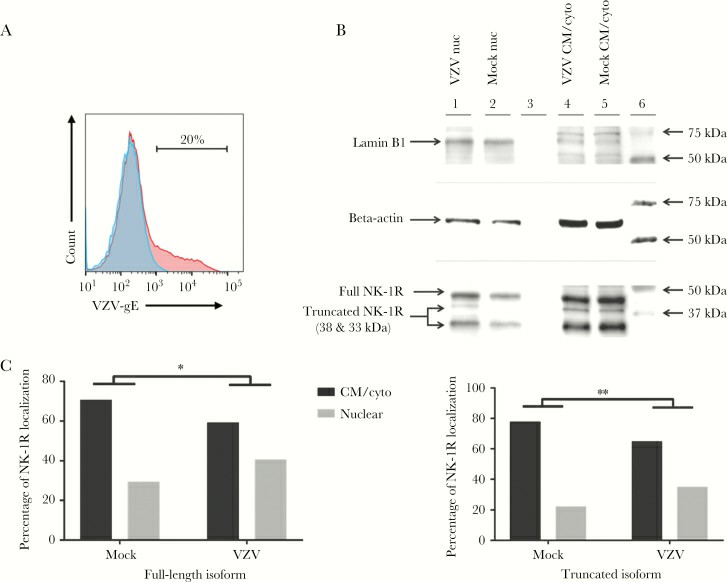 Fig. 3. Neurokinin-1 receptor localization in mock- and varicella zoster virus (VZV)–infected primary human spinal astrocytes, determined by Western blot (Bubak AN, Como CN, et al., 2018).
Fig. 3. Neurokinin-1 receptor localization in mock- and varicella zoster virus (VZV)–infected primary human spinal astrocytes, determined by Western blot (Bubak AN, Como CN, et al., 2018).
Ask a Question
Write your own review

