ONLINE INQUIRY

Human Astrocytes-midbrain (HA-mb)
Cat.No.: CSC-7832W
Species: Human
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The Human Astrocytes-midbrain cell line was isolated and cultured from human midbrain tissue. As part of the central nervous system, the midbrain lies between the brain and the brainstem, an important place for sensory information such as vision and hearing. Midbrain astrocytes live predominantly in the grey matter, close to neurons and capillaries. Known for their star-shaped shape, these cells carry many long processes, making it possible for them to link and wrap trillions of synapses between neurons. The fluorescent marker glial fibrillary acidic protein (GFAP) is widely used for staining these cells. As the dopaminergic neurons of the midbrain's substantia nigra are degenerated and dysfunctional in Parkinson's disease, and astrocytes are intimately associated with them, they have significant role to play in Parkinson's disease studies. The midbrain astrocytes also have multiple, distinct receptors and transporters, which can be useful targets for drugs. Thus, in neuropharmacological studies, the cell line of human midbrain astrocytes can be used to determine how drugs affect the midbrain neurotransmitter system.
α-Synuclein PFFs Induce NF-κB-Dependent Transcriptional Activity Associated with Astrocyte Activation
Parkinson's disease (PD) is a neurodegenerative condition characterized by the loss of midbrain dopamine neurons. Although pathogenesis remains obscure, misfolded or aggregated -synuclein proteins are associated with neurodegenerative mechanisms such as neuroinflammation and astrocyte activation.
Midbrain astrocytes play multiple roles in PD, initiating destructive and protective reactions during the course of the disease. Despite their crucial regulatory roles, it's still not entirely clear what cellular and molecular drives pathogenic astrocyte function. To evaluate the effects of α-synuclein aggregates on astrocyte activation, Chou et al. treated human midbrain astrocyte cultures with α-synuclein PFFs, known for inducing inflammatory activation and seeding α-synuclein aggregation. They conducted qPCR screening for transcripts linked to the A1 (neurotoxic) and A2 (neurotrophic) astrocyte states and general inflammatory markers. Experiments incorporated inhibitors targeting key inflammatory transcription pathways: BAY 11-7085 (inhibits NF-κB), Pyridone 6 (inhibits JAK/STAT), and SR 11302 (inhibits AP1). Overnight PFF treatment upregulated 8 of 11 A1 transcripts (Fig. 1a), with NF-κB blockade reversing this, while JAK/STAT and AP1 inhibition had limited impact. PFFs also upregulated 5 of 11 A2 genes (Fig. 1b) and inflammatory chemokines like CXCL10, verified by ELISA (Fig. 1f). α-Synuclein monomers didn't affect transcript levels. Responses were serum-independent, indicating α-synuclein PFFs induce a strong NF-κB-driven transcriptional response in astrocytes.
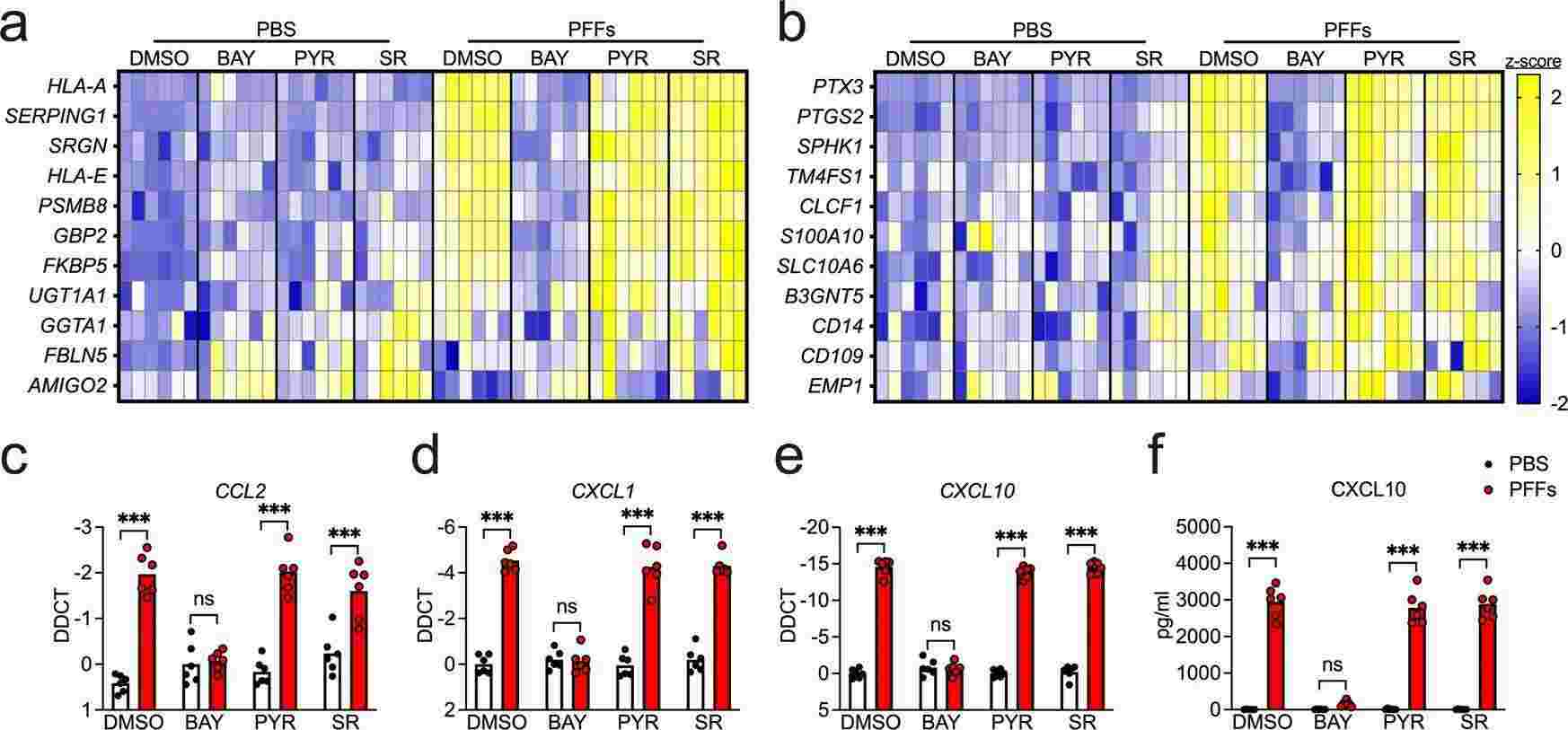 Fig. 1. α-synuclein PFFs induce NF-κB-dependent transcriptional activation in human midbrain astrocytes (Chou TW, Chang NP, et al., 2021).
Fig. 1. α-synuclein PFFs induce NF-κB-dependent transcriptional activation in human midbrain astrocytes (Chou TW, Chang NP, et al., 2021).
Secreted Factors from Dying Neurons Drive RIPK3-dependent Astrocyte Activation
Neuroinflammation is central to neurodegenerative diseases like Parkinson's and Alzheimer's. Astrocytes, as a type of glial cell, can take on a neurotoxic inflammatory state that exacerbates disease. RIPK3 is known to regulate neuroinflammation, but its role in astrocyte response to neuronal death is understudied. Chang's team used the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) model of Parkinson's disease to induce neuron death and observe RIPK3 signaling in astrocytes.
Transcriptomic profiling revealed that astrocytic RIPK3 promoted gene expression associated with neuroinflammation and movement disorders, and this coincided with significant engagement of damage-associated molecular pattern signaling. In mechanistic experiments, the results showed that factors released from dying neurons signaled through receptor for advanced glycation end products to induce astrocytic RIPK3 signaling, which conferred inflammatory and neurotoxic functional activity. To verify that inflammatory gene expression correlates with astrocyte activation, they tested the neurotoxic activity in human midbrain astrocytes of dying neuron-derived factors. Human midbrain astrocytes were treated with MPP+ NCM (MPP+: toxic metabolite of MPTP; NCM: neuronal conditioned media) for 24 hours, with or without a RIPK3 inhibitor. After washing and a 24-hour culture period, astrocyte-conditioned media (ACM) was added to SH-SY5Y cells in a 1:1 ratio with normal media (Fig. 2E). Astrocytes showed sustained transcriptional activation after MPP+ NCM removal (Fig. 3). ACM from MPP+ NCM-treated astrocytes caused about 80% cell death in SH-SY5Y cultures within 24 hours, while inhibiting astrocytic RIPK3 signaling prevented this neurotoxic activity (Fig. 2F). This shows that soluble factors from dying cells can trigger inflammation and neurotoxicity in astrocytes, relying significantly on astrocytic RIPK3.
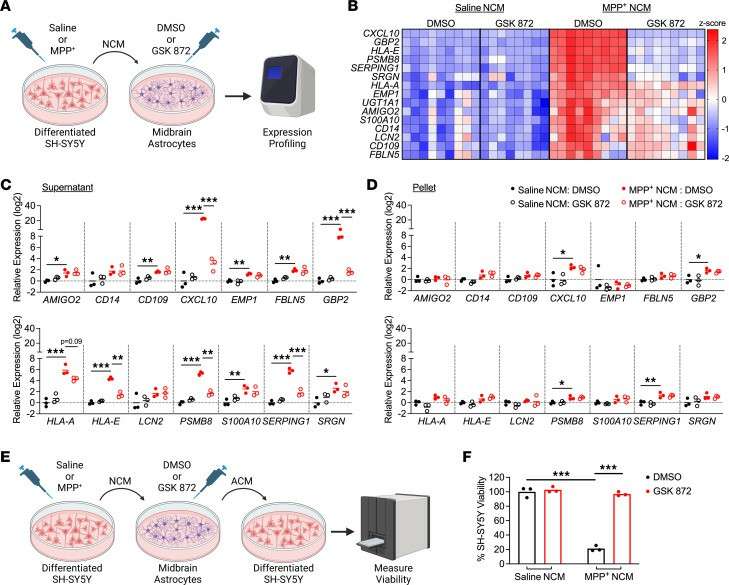 Fig. 2. Secreted factors from dying neurons drive RIPK3-dependent astrocyte activation (Chang NP, DaPrano EM, et al., 2024).
Fig. 2. Secreted factors from dying neurons drive RIPK3-dependent astrocyte activation (Chang NP, DaPrano EM, et al., 2024).
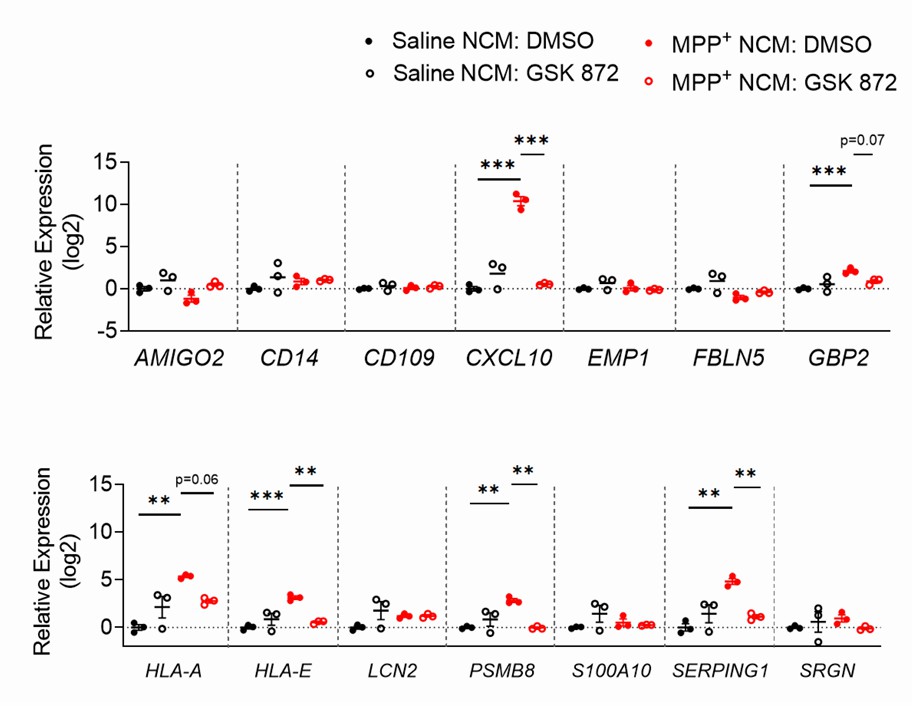 Fig. 3. Astrocytes maintain transcriptional activation for at least 24 hours following removal of MPP+ NCM (Chang NP, DaPrano EM, et al., 2024).
Fig. 3. Astrocytes maintain transcriptional activation for at least 24 hours following removal of MPP+ NCM (Chang NP, DaPrano EM, et al., 2024).
Ask a Question
Write your own review

