ONLINE INQUIRY

Human Astrocytes-hippocampal (HA-h)
Cat.No.: CSC-7833W
Species: Human
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Astrocytes are a major cellular constituent of the central nervous system (CNS), representing a complex and diverse cell population. Astrocytes have important functions in supporting the nervous system and play critical roles in the development and function of the CNS. Specifically, astrocytes provide functional support, such as maintaining the blood-brain barrier, regulating regional blood flow, providing nutrients and metabolic factors to neurons, recovering neurotransmitters (uptake and release), maintaining the internal environment balance of neural networks, and regulating the formation and transmission of synapses. Astrocytes can also regulate the activation, proliferation, and survival of T cells by producing chemokines. Due to their vital role in neurophysiological processes, disruption of astrocyte function is associated with many neurological disorders, including Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple sclerosis, and amyotrophic lateral sclerosis. Therefore, elucidating the mechanism of the functional diversity of astrocytes and recognizing the internal and external factors of astrocytic heterogeneity have become a hot topic.
Most of our understanding of astrocyte biology comes from in vitro examinations performed on human and rodent astrocytes. Studies of astrocytes in cell culture have provided investigators with the opportunity to predict astrocyte gene expression in the brain, to identify factors that regulate astrocytic expression of cell adhesion molecules and chemokines, and to establish in vitro models of the blood-brain barrier that can be used for CNS drug discovery and development.
Table 1. Comparison between the different cell culture techniques for astrocyte research (Preato, André Maciel, et al. 2024).
| Culture Type | Advantages | Limitations |
| Primary Culture | Low cost and simple methodology. | Usually uses serum and easily induces reactive phenotypes. High percentage of cell senescence with passages; difficult to scale up; prone to contamination. |
| Cell Lines | Easy to obtain; allow multiple passaging; easy to manipulate; reduces animal usage. | Cancer cell phenotype; expression profile is different from astrocytes in vivo. |
| Immortalized Astrocytes | Intended to maintain features of primary cells; increased adaptability to passaging and freeze-thawing; easier to manipulate. | Similar to primary cell culture; genomic integration promotes cellular changes. |
| iPSC-Derived Astrocyte | Relevant to disease studies (patient- derived); good proliferation; serum-free; can be grafted; reduced animal usage. | High cost; long-term culture; can present mutations. |
| 3D Culture/Organoids | Mimics in vivo conditions, allows analysis of complex interactions between different cell types. | High cost; difficult to reproduce results; difficult nutrient diffusion. |
Characterization of Primary Human Hippocampal Astrocyte Cell Culture Following Exposure to Hypoxia
The study aimed to understand the characterization of human hippocampal astrocytes following hypoxia exposure. They choose 15 min as the time point and expose astrocyte cells to various oxygen levels (15% oxygen, 10% oxygen, 5% oxygen and 3% oxygen).
Control and hypoxia cells were stained with Annexin V-FITC and then observed under a fluorescence microscope. Cells with high fluorescence intensity due to chromatin condensation or nuclear fragmentation are considered as apoptotic cells. The number of high-fluorescent condensed or fragmented nuclei was counted randomly in each group. Results are expressed as the percentage of condensed or fragmented nuclei relative to the total number of cells counted for each experimental condition (Fig. 1 and 2).
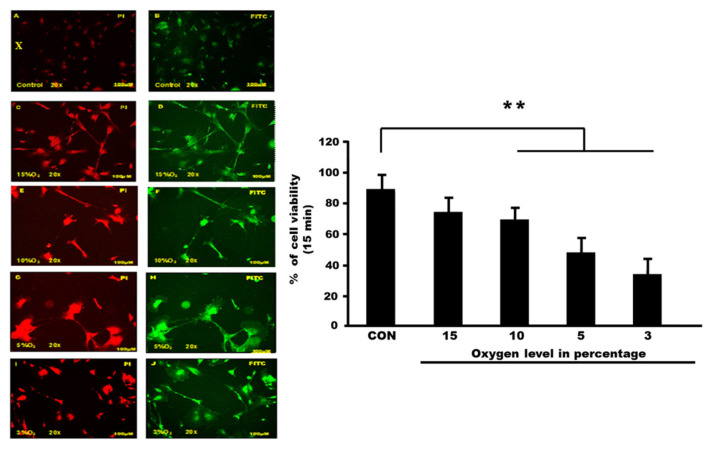 Fig. 1. Apoptosis detection via Annexin V-FITC staining (Nazli, Nurul Atikah Nor, et al. 2023).
Fig. 1. Apoptosis detection via Annexin V-FITC staining (Nazli, Nurul Atikah Nor, et al. 2023).
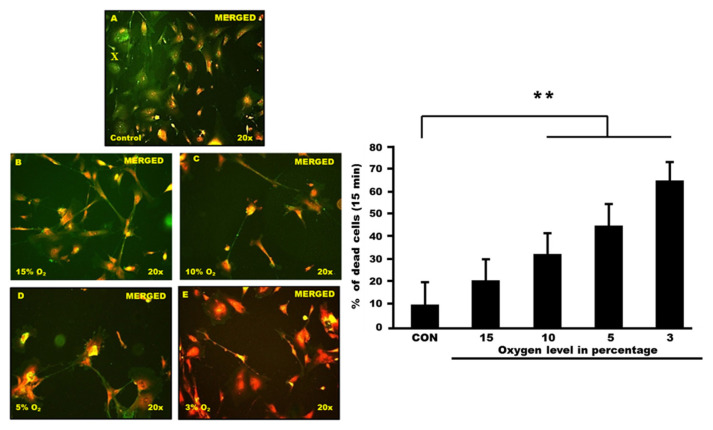 Fig. 2. PI and FITC staining of human hippocampal astrocyte cells (Nazli, Nurul Atikah Nor, et al. 2023).
Fig. 2. PI and FITC staining of human hippocampal astrocyte cells (Nazli, Nurul Atikah Nor, et al. 2023).
Palmitic Acid (PA) Has a Greater Impact on The Cell Viability of Male Human Astrocytes
The MTT assay was initially used to evaluate cell viability in human male and female astrocytes. The results indicated that cell viability was influenced by both sex and treatment. Concentrations of 0.5 mM and 1 mM demonstrated significant differences in cell viability after 24 h (Fig. 3A). We stained DNA damage using the propidium iodide (PI) assay to confirm this outcome, which helps identify living and dead cells. At a concentration of 1 mM, male astrocytes displayed a 20% increase in PI fluorescence in comparison to their female counterparts (Fig. 3B).
Next, we investigated whether PA exposure results in apoptotic cell death in astrocytes. Fig. 3C shows that most male and female cells were Annexin V-/PI+, indicating that necrosis accounted for most of the death. Accordingly, we conducted further analysis on the subset of cells testing positive for both Annexin V and PI, as shown in Fig. 3D. At a concentration of 1 mM, a 20% higher rate was observed in male astrocytes compared to female cells, with sex identified as a significant factor in the outcome. These findings suggest that sex is a critical variable, revealing a differential response in the presence of PA, with male astrocytes exhibiting more pronounced detrimental effects than female cells.
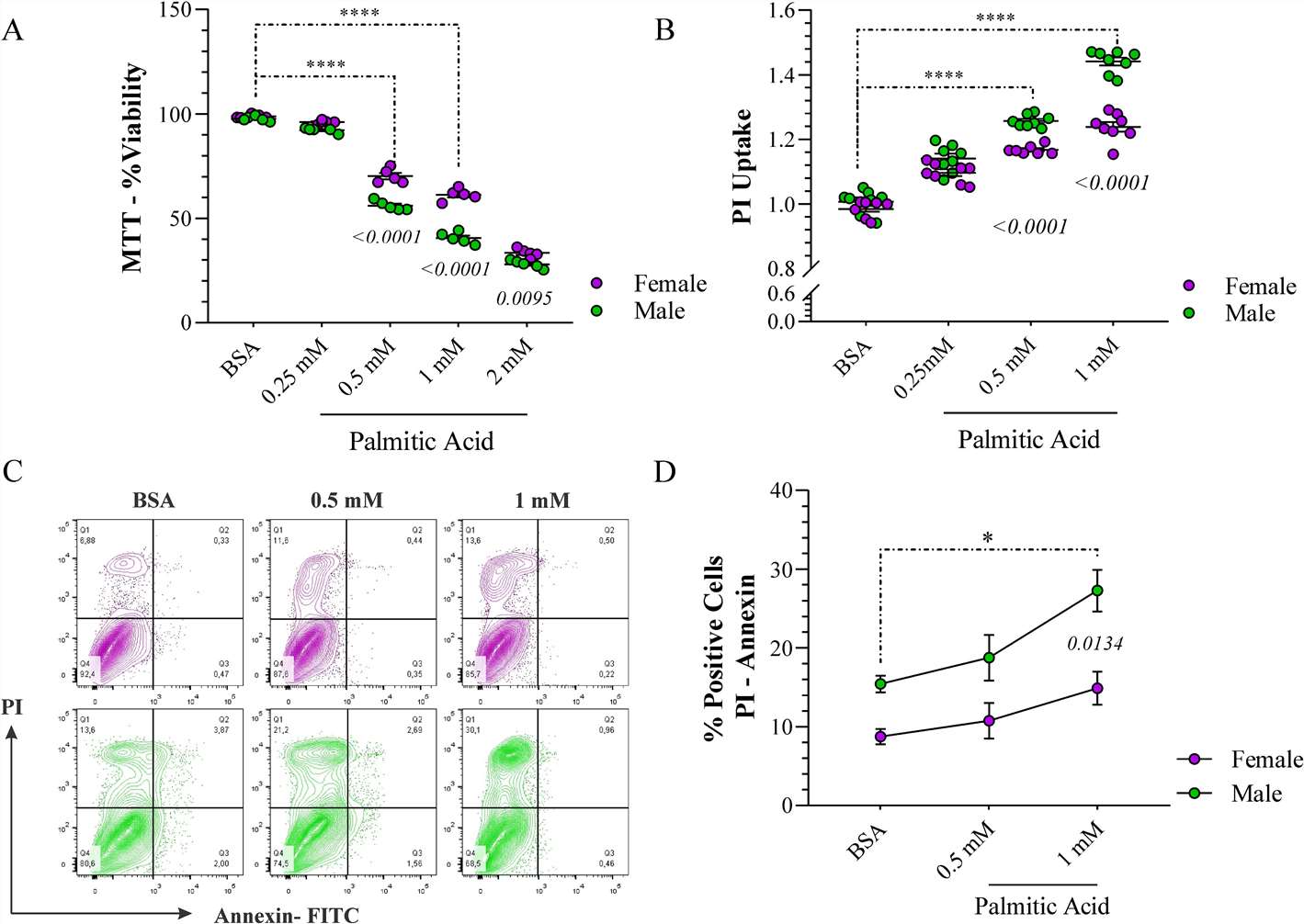 Fig. 3. The cytotoxic effects of palmitic acid (PA) on human astrocytes (Hidalgo-Lanussa, Oscar, Janneth González Santos, and George E. Barreto. 2024).
Fig. 3. The cytotoxic effects of palmitic acid (PA) on human astrocytes (Hidalgo-Lanussa, Oscar, Janneth González Santos, and George E. Barreto. 2024).
Ask a Question
Write your own review


