ONLINE INQUIRY

Human Astrocytes-brain stem (HA-bs)
Cat.No.: CSC-7835W
Species: Human
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The human astrocytes-brain stem (HA-bs) cell line derives from the human brainstem, an area located at the intersection of the brain and spinal cord that regulates key processes in life, including breathing and heartbeat. These cells are central to the central nervous system. With their long processes, astrocytes form a scaffolding system between neurons that gives neurons structural support. By the nutrition aspect, astrocytes produce and release certain neurotransmitters, and also harbor different neurotransmitter receptors. They support neurons' metabolic demands by stabilizing their surrounding ionic environment. Moreover, by secreting neurotransmitters, astrocytes can regulate the synaptic activity of neurons and therefore affect the transmission of neural signals.
Astrocytes have also been shown to be important for memory storage and retrieval – they collaborate with neurons to enhance memory generation and recall. The astrocytic impairment can result in stroke and other neurologic conditions. Thus, the HA-bs cell line can be used to create disease models, study the pathogenesis of neurodegenerative and other conditions, and support the discovery of novel therapeutic approaches. And in the context of neural regeneration, the HA-bs cell line plays an important role, too, because it can be applied to research how astrocytes help to regenerate and repair after injury to the nervous system.
Regional Astrocyte Localization Cues are Regulated by Cytokines
Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease of the central nervous system (CNS), with symptoms and outcomes influenced by inflammatory lesion locations. While T cell cytokines are known to regulate leukocyte entry into specific CNS regions, the molecular mechanisms of this regulation remain unclear. Williams's team used in vitro and in vivo models to analyze murine and human astrocytes' responses to Th1 and Th17 cytokines, elucidating how astrocytes exhibit region-specific responses to T cell cytokines and affect CNS region-specific neuroinflammation. Astrocytes envelop the CNS endothelium, maintain barrier properties, react to cytokines, and express molecules like CXCL12, CXCR7, and VCAM-1, key in T cell localization. Previous research showed CXCL12 regulation by CXCR7 affects spinal cord infiltration and EAE severity, with astrocytic CXCR7 also modulating extracellular CXCL12 levels.
To study cytokine effects on astrocytic T cell localization cues, human brain stem and spinal cord astrocytes (Fig. 2a and b) were exposed to cytokines steering inflammation towards the brain stem or spinal cord, assessing VCAM1 and CXCR7 mRNA (Fig. 1f and g). IL-1β reduced VCAM1 mRNA in both CNS regions but lowered CXCR7 only in spinal cord astrocytes (Fig. 2c and d). Contrarily, IL-17 and TNFα elevated VCAM1 in brain stem over spinal cord astrocytes (Fig. 2c), while IFNγ significantly increased CXCR7 in spinal cord astrocytes (Fig. 2d). Although GM-CSF is linked to EAE and MS inflammation, it didn't show notable regional differences in VCAM1 transcripts post-treatment. To assess if regional baseline expression levels of cytokine receptors cause different cytokine responses, they conducted qRT-PCR on primary human astrocyte receptors. Their findings showed that untreated human brain stem astrocytes exhibit higher IL17R expression, while spinal cord astrocytes have elevated baseline IFNGR1 levels (Fig. 2e). These results indicate that Th1 cytokines affect the CXCL12/CXCR7 chemokine axis in spinal cord astrocytes, whereas Th17 cytokines upregulate VCAM-1 in brain stem astrocytes. These differences may stem from heightened receptor expression in these CNS regions.
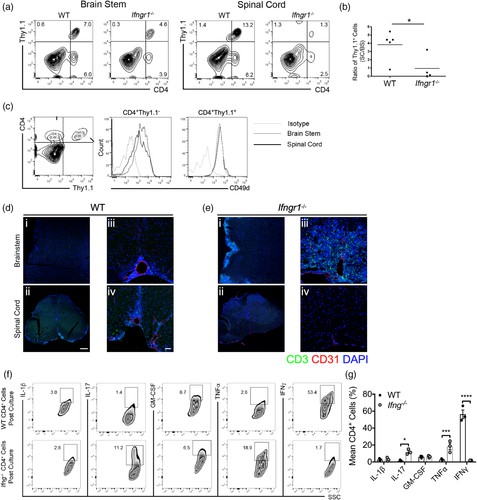 Fig. 1. Cytokines dictate regional T cell trafficking within the CNS (Williams JL, Manivasagam S, et al., 2020).
Fig. 1. Cytokines dictate regional T cell trafficking within the CNS (Williams JL, Manivasagam S, et al., 2020).
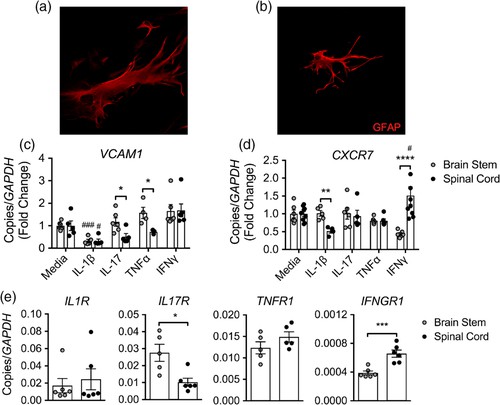 Fig. 2. Cytokines mediate regional localization cues on astrocytes (Williams JL, Manivasagam S, et al., 2020).
Fig. 2. Cytokines mediate regional localization cues on astrocytes (Williams JL, Manivasagam S, et al., 2020).
Cellular Senescence in Cultured Human Brainstem Astrocytes: Effect of Oxidative Stress
Aging increases the risk of neurodegenerative and cardiovascular diseases, linked to senescent cells that accrue in brain areas including the brainstem. Although neurons don't senesce, nearby astrocytes do, impacting autonomic cardiovascular control. Leibhart et al. developed an in vitro model using cultured human brainstem astrocytes to investigate brainstem cellular senescence, specifically focusing on the effects of oxidative stress.
p16, p21 and p53 are the major cyclin dependent kinase inhibitors which are well documented senescent cell markers. They detected the mRNA level these cell markers after introduced human brainstem astrocytes to hydrogen peroxide treatments. IL6 is a proinflammatory cytokine and is a major SASP factor that is upregulated in senescence. Lamin B1 is a gene that encodes Lamin proteins which are a part of the nuclear envelope. CXCL1 is a chemokine that is responsible for recruitment of neutrophils and oligodendrocytes. In my study, they observed a non-significant increase in the levels of p16, p21 and p53. The levels of IL6 were found to be significantly higher in the H2O2 treated astrocytes as compared to the control (untreated) astrocytes (Fig. 3). A non-significant decrease was observed in the levels of Lamin B1 in the H2O2- treated astrocytes (Fig. 3). A significant downregulation was seen in the levels of CXCL1 in the treated cells (Fig. 3). These results indicated that the oxidative stress induces senescence in cultured human brain stem astrocytes as indicated by higher levels of p16 and increase in the IL-6. The significant decrease in the levels of LaminB1 implies damage to the nuclear lamina indicating senescence.
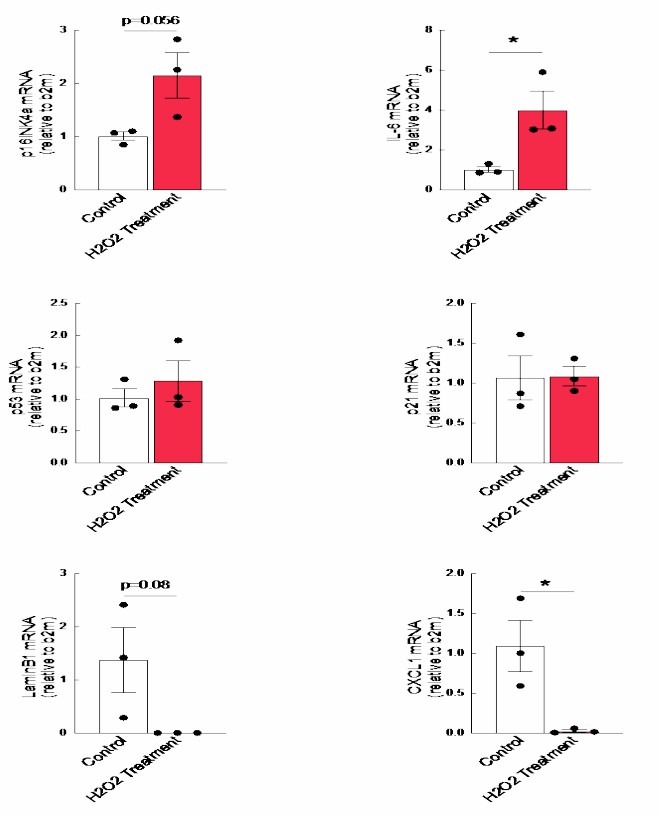 Fig. 3. There are increased levels of p16 and IL6 after hydrogen peroxide treatment in the cultured astrocytes accompanied with decreased levels of LaminB1 (Leibhart Z and Subramanian M, 2019).
Fig. 3. There are increased levels of p16 and IL6 after hydrogen peroxide treatment in the cultured astrocytes accompanied with decreased levels of LaminB1 (Leibhart Z and Subramanian M, 2019).
Ask a Question
Write your own review

