ONLINE INQUIRY

Human Astrocytes (HA)
Cat.No.: CSC-7836W
Species: Human
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The central nervous system tissues (brain and spinal cord) are where most human astrocytes grow. Creative Bioarray mainly derives its human astrocytes from the cerebral cortex. In terms of the content of glial filaments and cell processes, there are two kinds of astrocytes. The first is fibrous astrocytes, mostly found in the cortical regions of the brain and spinal cord. These cells have long and thin processes, with fewer branches, and they have a cytoplasm full of glial filaments (hence the term "spider cells"). The second type is protoplasmic astrocytes, more typically associated with gray matter areas, with very short, thick and well-branched processes. The processes of fibrous astrocytes are like long tubes, while those of protoplasmic astrocytes are light and sheet-like, sometimes wrapping around neurons and synapses without ever reaching into the synaptic cleft.
Astrocytes are involved in homeostasis of the central nervous system: neurotransmitter cycling, ion exchange, energy metabolism, synaptogenesis and synaptic transmission, as well as the blood-brain barrier. When central nervous system inflammation occurs, astrocytes are both pro- and anti-inflammatory and they partner with other cells and molecules to release cytokines, chemokines and neuroregulatory molecules. This importance of astrocytes is coming to be understood in human disease because they are involved in a variety of neurodegenerative disorders including Alzheimer's and Parkinson's. In addition, astrocyte research helps us learn more about chronic pain, neurodevelopmental conditions and nervous-system healing after brain injury.
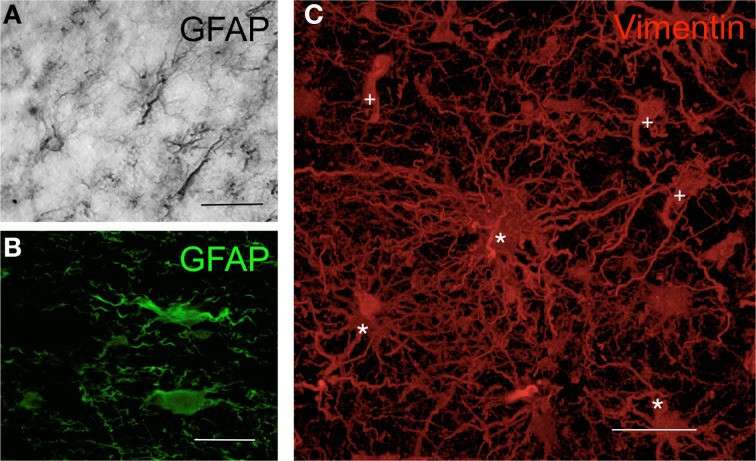 Fig. 1. Morphology of human astrocytes by immunohisto-chemistry (Barcia CS, Mitxitorena I, et al., 2013).
Fig. 1. Morphology of human astrocytes by immunohisto-chemistry (Barcia CS, Mitxitorena I, et al., 2013).
Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli
Astrocytes, once regarded as passive brain cells, are now active members of the central nervous system (CNS) with different functional identities. Szpakowski's team aimed to examine whether pro-inflammatory or anti-inflammatory human astrocytes produce inflammatory chemokines and neurotrophic factors that contribute to CNS pathologies.
GFAP was increased in astrocytes that were exposed to the microglia proinflammatory cytokine cocktail (TNF-α/IL-1a/C1q) compared with unstimulated cells assessed by flow cytometry. The levels of surface receptor expression were also not changed in the CD80 or CD86 densities. However, the CD83 level in cells treated with TNF-α/IL-1a/C1q was significantly higher than in controls (Fig. 1). The stimulation with TNF-α/IL-1a/C1q resulted in high levels of the chemokines CCL1, CXCL1, CXCL10 and CXCL13 and proinflammatory IL-1, which were not present in unstimulated or anti-inflammatory cytokine-treated astrocytes (Fig. 2). The unstimulated astrocytes generated an abundance of PDGF-A, and were not impacted by IL-4 or IL-10. BDNF was detected but unaffected by the conditions (Fig. 3). GDNF appeared in one donor culture, and β-NGF was undetected. These results confirm that astrocytes differentially respond to pro- and anti-inflammatory stimuli, especially to inflammatory cytokines TNF-α, IL-1a, and C1q, suggesting their role in leukocyte recruitment.
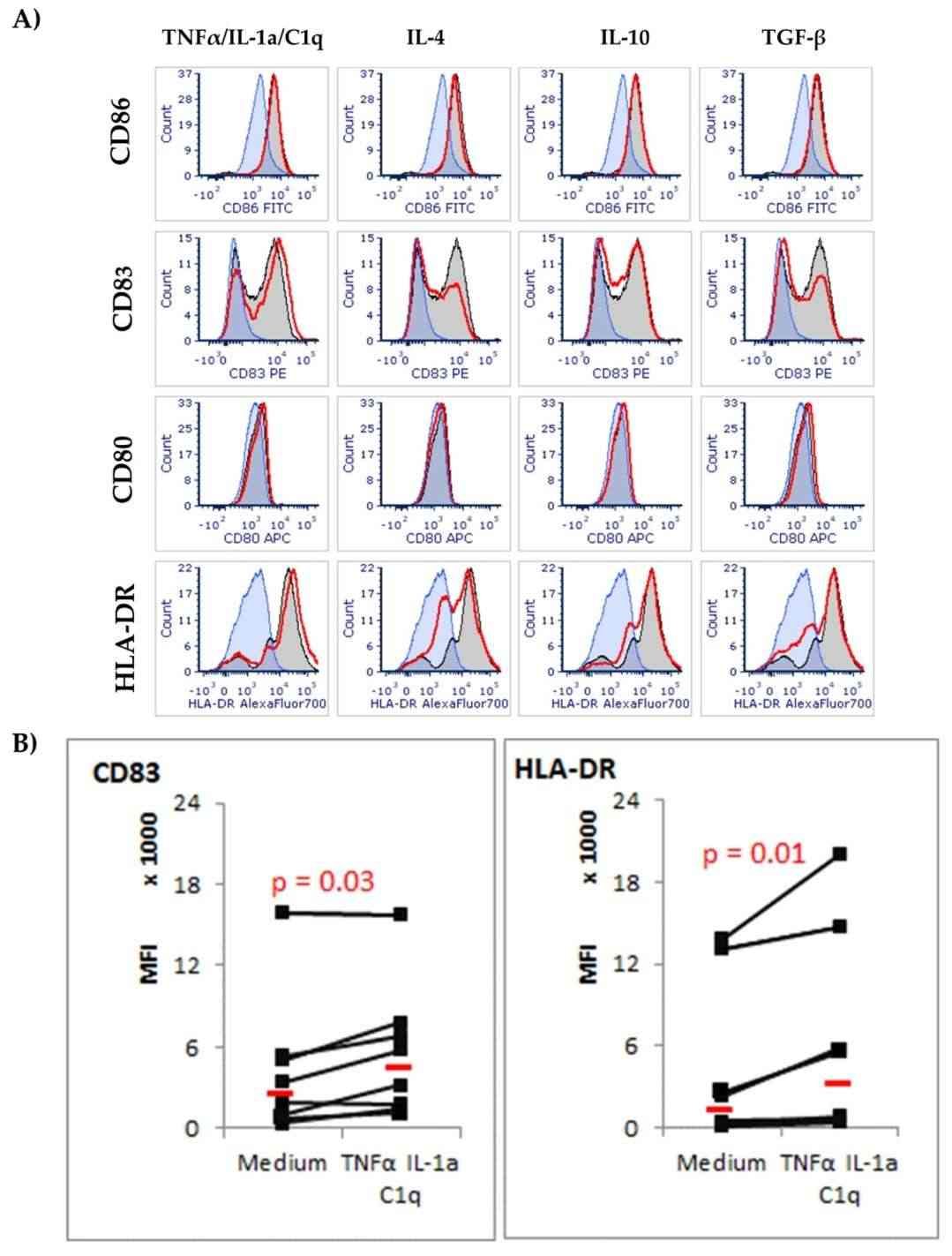 Fig. 1. Expression of surface co-stimulatory CD86, CD83, CD80, and MHC class II (HLA-DR) molecules on astrocyte cells in response to cytokine environment (Szpakowski P, Winiarek DK, et al., 2022).
Fig. 1. Expression of surface co-stimulatory CD86, CD83, CD80, and MHC class II (HLA-DR) molecules on astrocyte cells in response to cytokine environment (Szpakowski P, Winiarek DK, et al., 2022).
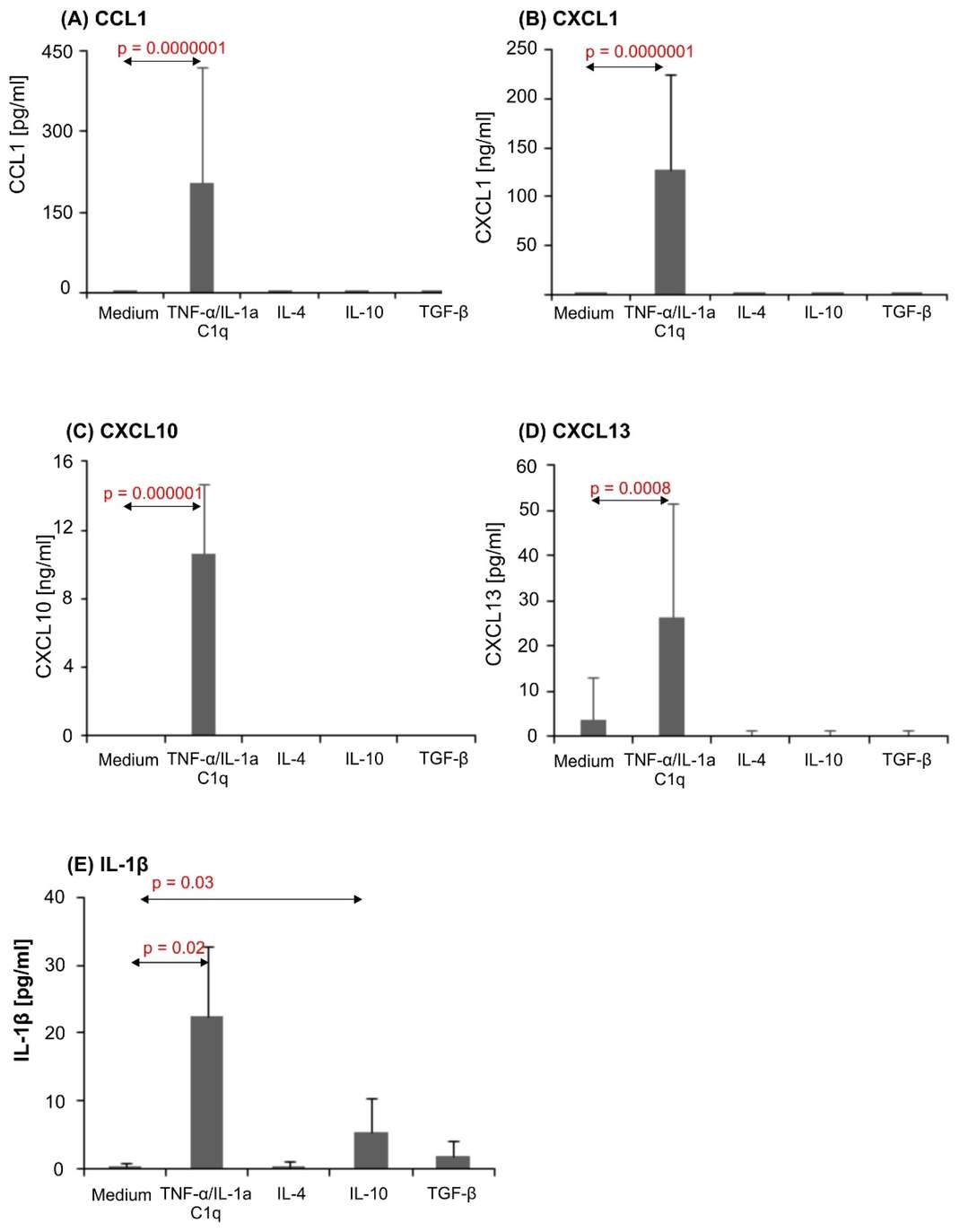 Fig. 2. CCL1 (A), CXCL1 (B), CXCL10 (C), CXCL13 (D), and IL-1β (E) production in human astrocyte cultures (Szpakowski P, Winiarek DK, et al., 2022).
Fig. 2. CCL1 (A), CXCL1 (B), CXCL10 (C), CXCL13 (D), and IL-1β (E) production in human astrocyte cultures (Szpakowski P, Winiarek DK, et al., 2022).
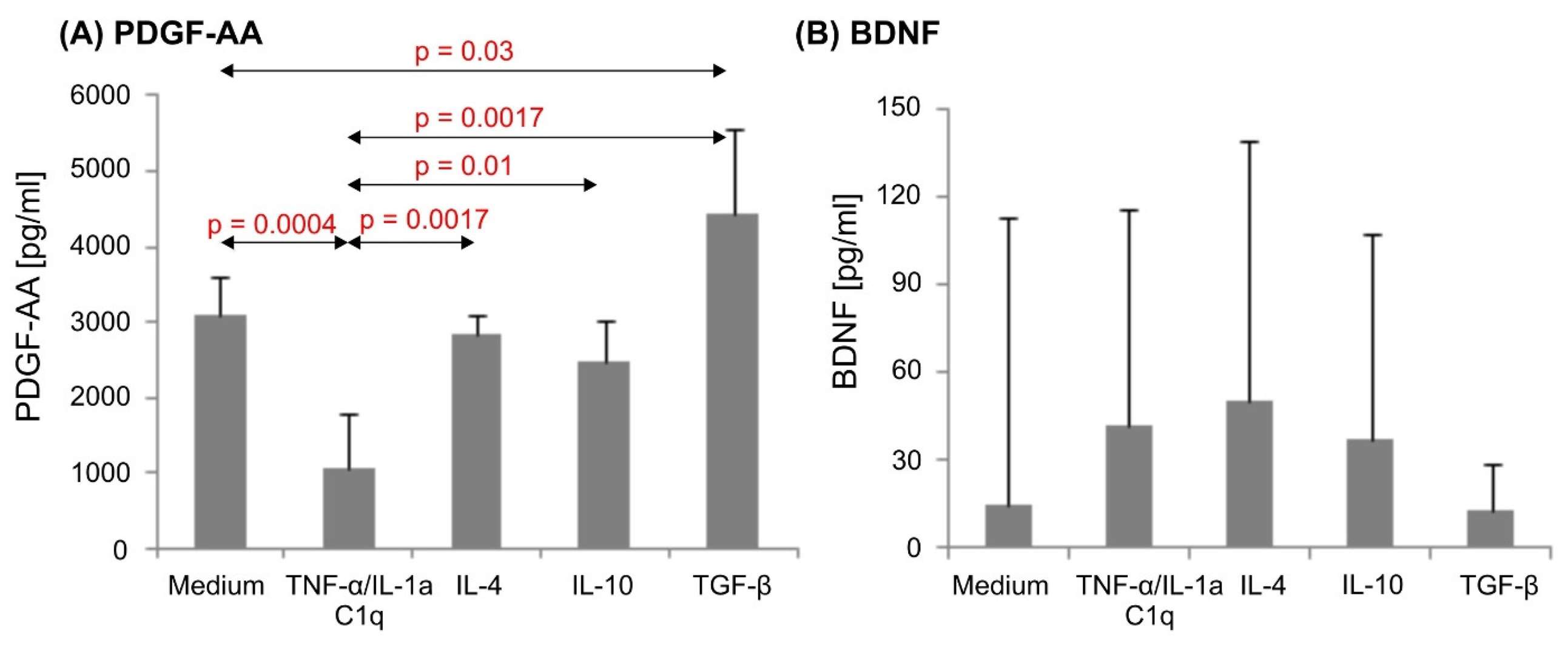 Fig. 3. PDGF-AA (A) and BDNF (B) production in human astrocyte cultures (Szpakowski P, Winiarek DK, et al., 2022).
Fig. 3. PDGF-AA (A) and BDNF (B) production in human astrocyte cultures (Szpakowski P, Winiarek DK, et al., 2022).
Levels of Viral Infection Differ between the Asian and African ZIKV Strains in Human Astrocytes
Zika virus (ZIKV), a flavivirus, is known for causing neurodevelopmental disorders and has notably emerged in regions like French Polynesia and Brazil. It's transmitted mainly by Aedes mosquitoes, with astrocytes as primary targets in the CNS. However, the mechanisms of ZIKV's entry and pathology remain unclear. Ojha et al. compared the level of infectivity and the release of inflammatory molecules among three isolated strains of ZIKV representing the African and Asian lineages and determine the mechanisms of their responses in primary human astrocytes.
ZIKV infection in human astrocytes was assessed using immunofluorescence, RT-PCR, and western blot. In astrocytes, different infectivity levels were found among ZIKV strains, with Asian strains (PRVABC59 and R103451) showing higher infectivity than the African strain (MR766) (Fig. 4A-C, Fig. 5A). Infected microglia showed similar patterns. Plaque assays revealed peak viral titers at 48 hpi, while intracellular RNA levels were higher than viral progeny in supernatants, highlighting differences between RNA presence and active virus release (Fig. 4D). Despite high infectivity, astrocytes released fewer viral particles than microglia (Fig. 5D). To explore viral entry in human glial cells, they studied TAM receptors Tyro3, AXL, and MERTK in astrocytes. ZIKV infection didn't change AXL levels but slightly increased Tyro3 expression with Asian and African strains (Fig. 4E-G). The AXL receptor's role was tested using R428, which decreased ZIKV (MR766 and PRVABC59) cell entry. For the Honduras strain (R103451), entry decreased only at high inhibitor concentrations (Fig. 4H, Fig. 5E and F). siRNA silencing of AXL showed similar results (Fig. 5H and I). These findings suggest strain-dependent AXL role differences. Despite decreased IFN-β signaling and increased IP-10 in PRVABC59-infected astrocytes treated with R428, ZIKV entry persisted, implying alternative pathways. Tyro3 inhibition with BMS777607 had no effect, indicating ZIKV entry is Tyro3-independent (Fig. 5J and K).
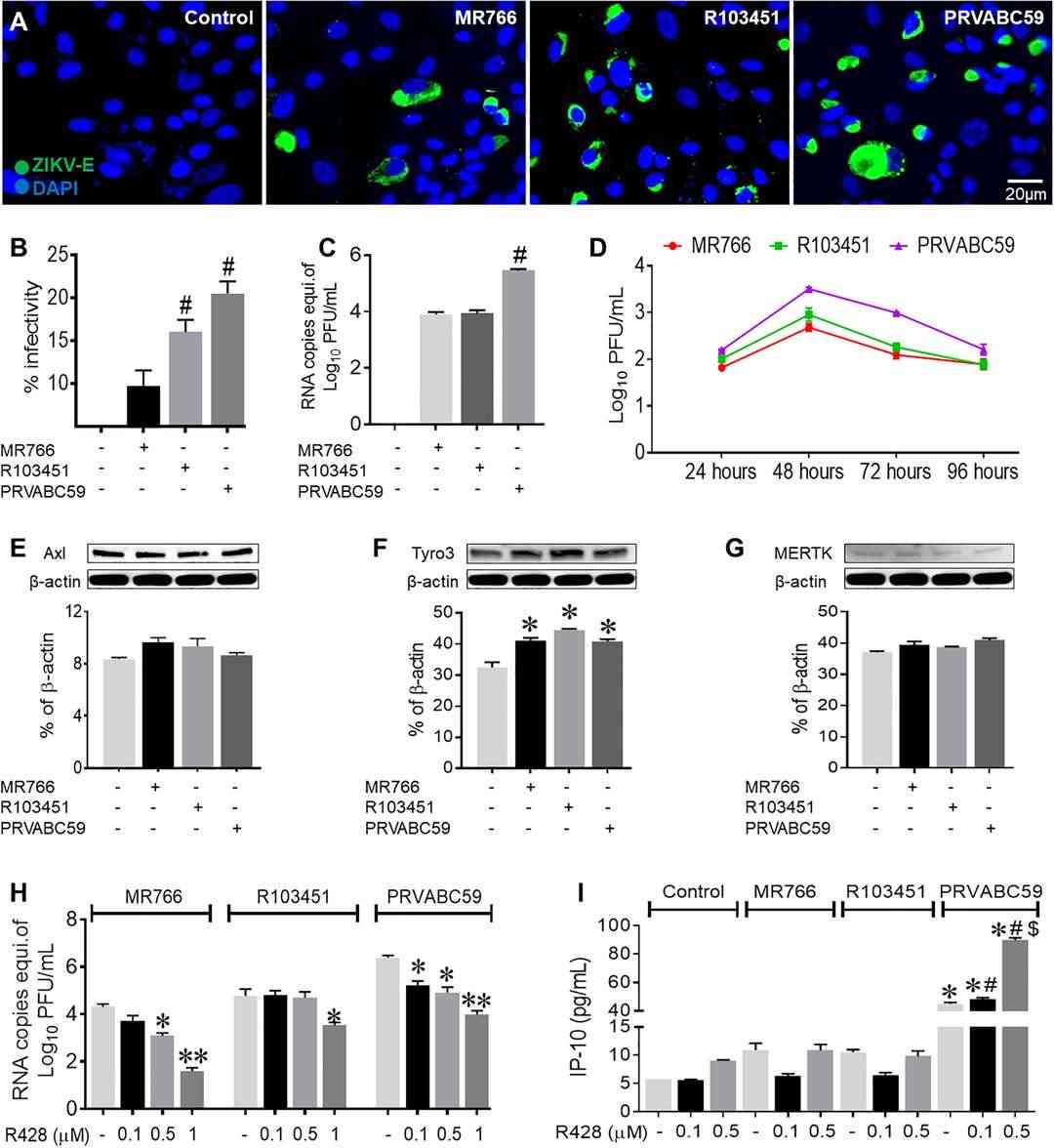 Fig. 4. Asian and African strains of ZIKV have differential levels of infectivity in human astrocytes (Oiha CR, Rodriguez M, et al., 2019).
Fig. 4. Asian and African strains of ZIKV have differential levels of infectivity in human astrocytes (Oiha CR, Rodriguez M, et al., 2019).
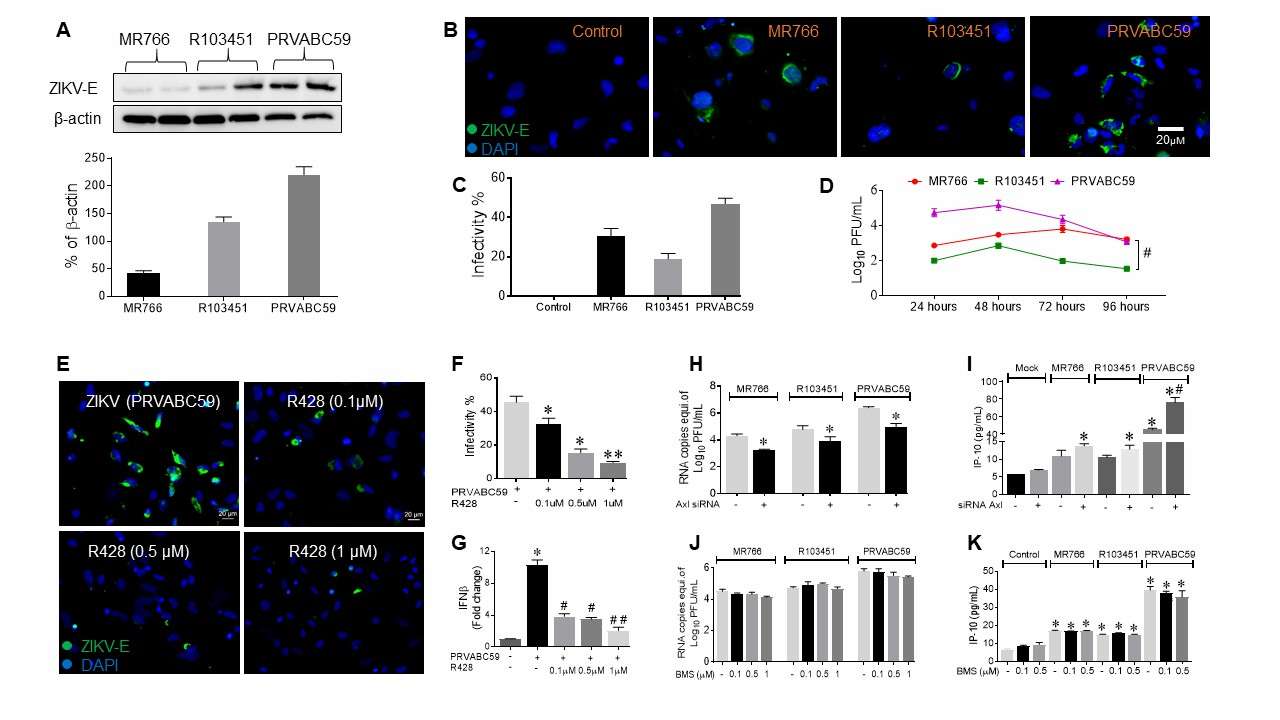 Fig. 5. ZIKV infects human microglial cells and utilizes AXL receptor for cell entry (Oiha CR, Rodriguez M, et al., 2019).
Fig. 5. ZIKV infects human microglial cells and utilizes AXL receptor for cell entry (Oiha CR, Rodriguez M, et al., 2019).
Human Astrocytes from Creative Bioarray are guaranteed through 10 population doublings, express GFAP by immunofluorescence.
Ask a Question
Write your own review

