ONLINE INQUIRY
Human Adrenal Fibroblasts (HAdF)
Cat.No.: CSC-7844W
Species: Human
Source: Adrenal Gland
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human adrenal fibroblasts (HAdF) are mesenchymal cells from the embryonic mesoderm, and are a vital cell type within adrenal tissue. These cells are primarily located in the capsule and cortex regions of the adrenal glands. They usually have long spindle or cuneiform forms with several protrusions and rolled-up nuclei. The human adrenal is also biologically diverse. They can produce and release many proteins, enzymes and other bioactive compounds essential to the normal physiology and function of the adrenal glands. Fibroblasts support this.
Fibroblasts produce and secrete structure proteins of the ECM, type I collagen, elastin, adhesive proteins like fibronectin and laminin, as well as matrix components. Structural proteins increase ECM rigidity or plasticity; adhesive proteins anchor cells to the ECM. This carries nutrients to tissues where the blood vessels are too small and is a conduit for cell-to-cell communication. When tissues are damaged, fibroblasts repair the wound. They also recruit inflammatory and immune cells, and produce cytokines and chemokines that propel inflammatory response. Yet unregulated fibroblast growth defines pathological fibrosis and its accompanying diseases. Adrenal fibroblasts, then, are models of physiological and pathological processes of the adrenal glands, such as adrenal tumors, Cushing's syndrome, and adrenal cortical insufficiency.
Optimizing the Co-Culture Screening System to Monitor Cell Death in Tumor-Stroma Interactions
Selecting the most beneficial drug or combination for a patient remains a central challenge in medicine, especially in cancer therapy. Prior studies mainly focused on tumor genomics but failed to fully explain drug response variability. Tumor heterogeneity necessitates tailored treatments, but traditional genetic approaches don't fully account for variations in drug response. Landry's team focuses on the tumor microenvironment, particularly tumor-stroma interactions.
The mixed coculture assay was developed to investigate these interactions, revealing unexpected diversity in tumor-stroma interactions. Cancer cells were genetically modified to express GFP, enabling rapid and quantitative measurement of drug response dynamics. Microscopy revealed that sensitivity to both targeted and cytotoxic therapies was inhibited by coculture with HADF (human fibroblast harvested from the adrenal gland) (Fig. 1A and B). The fluorescence plate reader only captured the fibroblast-dependent inhibition of erlotinib-induced growth arrest but failed to capture the inhibition of cytotoxic therapies (Fig. 1C). Additionally, the plate reader failed to capture the significant cell death observed following camptothecin exposure (Fig. 1C); the stability of GFP fluorescence may be the reason (Fig. 1D and E). Based on these results, they refined the coculture screening to optimize the measurement of drug-induced cell death. They used JC-1 dye, which reflects mitochondrial integrity and indicates whether cells are undergoing apoptosis (Fig. 2A). To evaluate JC-1's effectiveness in measuring cell death in coculture, they tested BT-20 cells with camptothecin, with or without HADF. Before drug exposure, red fluorescence was seen in BT-20 but not in HADF, indicating no dye transfer between cells (Fig. 2B). After 96 hours, the BT-20 cells showed reduced JC-1 red fluorescence, indicating compromised mitochondrial integrity (Fig. 2C). The JC-1 red fluorescence was sensitive enough to detect BT-20 cell death alone and the protective effect of HADF in coculture (Fig. 2D).
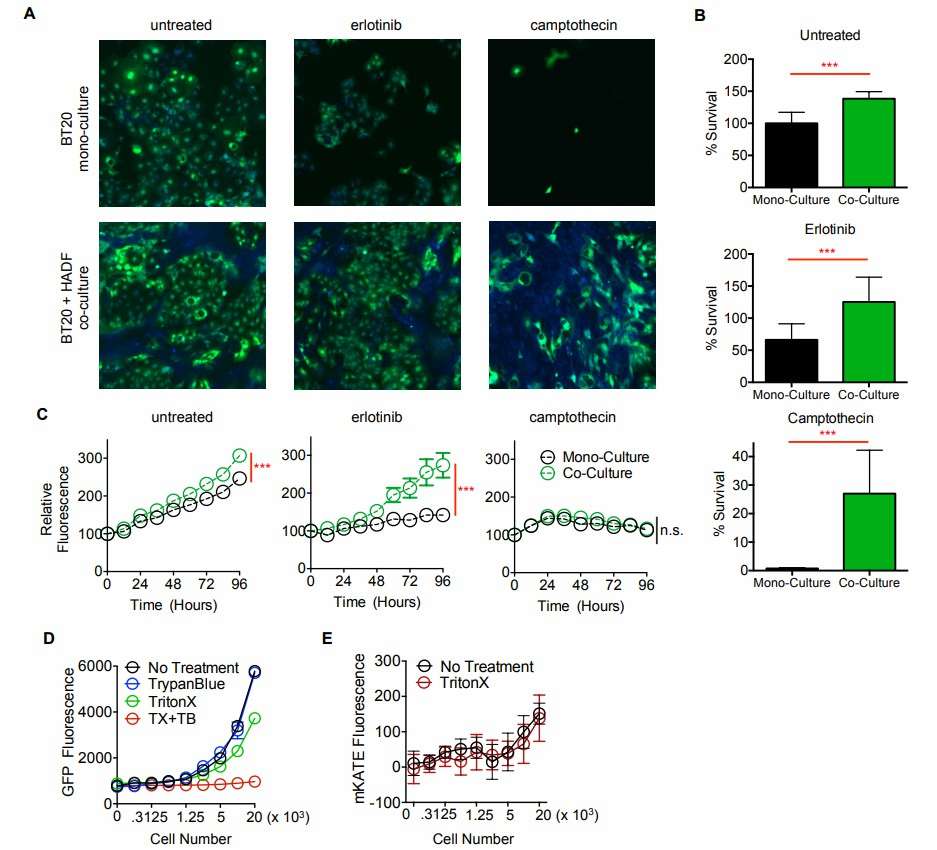 Fig. 1. GFP-based measurements do not accurately report cell death (Landry BD, Leete T, et al., 2018).
Fig. 1. GFP-based measurements do not accurately report cell death (Landry BD, Leete T, et al., 2018).
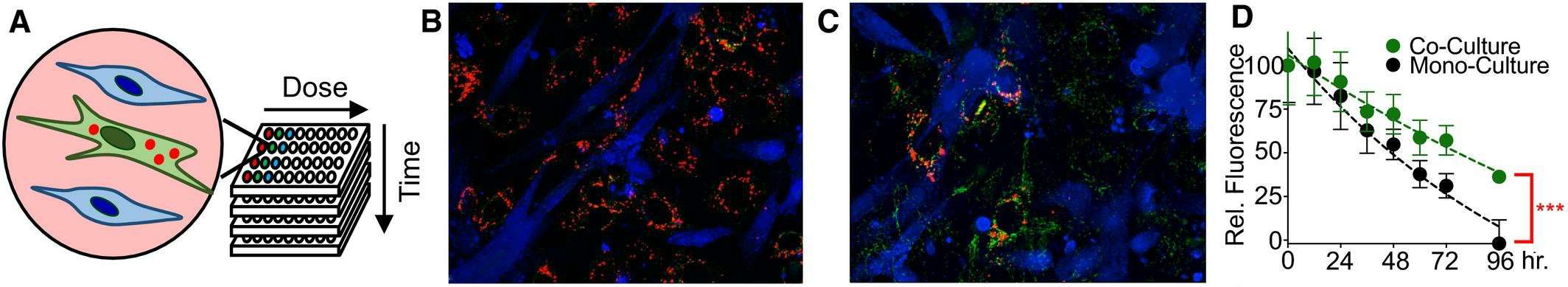 Fig. 2. Coculture screen to identify tumor–stroma interactions reveals divergent interactions between fibroblasts and TNBC cells (Landry BD, Leete T, et al., 2018).
Fig. 2. Coculture screen to identify tumor–stroma interactions reveals divergent interactions between fibroblasts and TNBC cells (Landry BD, Leete T, et al., 2018).
Single-Nucleus and Spatial Transcriptome Analysis of Adrenal Cortex and Adrenal Medulla (Fibroblasts and Connective Tissue Cells)
The human adrenal gland, a complex endocrine organ, maintains homeostasis via steroid hormones and catecholamines. Prior studies on adrenal renewal focus mainly on animal models or fetuses, leaving a gap in adult human understanding. With adrenal tumors' incidence rising with age, especially benign adrenocortical adenomas (ACAs), there's an urgent need to comprehend normal adrenal homeostasis.
Altieri's team utilized single-nuclei RNA sequencing and spatial transcriptomics to provide a detailed cellular genomics analysis of adult human adrenal cortex, including spatial data, to understand normal adrenal function and adrenocortical tumorigenesis. Transcriptome data analysis of adrenal fibrocytes and connective tissue cells revealed that, the fibroblasts and connective tissue cluster were characterized by cells that highly expressed collagen markers, such as COL1A2 and COL4A1, and markers of the extracellular matrix, such as MGP, FBLN1, LAMA2 and CCN1 (Fig. 3D). Using IHC, we confirmed the protein expression of COL1A2 in the adrenal capsule and MGP in the extracellular matrix and fibroblasts. Pathway analyses validated the satellite cluster annotations, revealing enrichment of cluster-specific gene sets, such as phagosome and NF-kappa B signaling (Fold Enrichment (FE) > 4 for both pathways) for myeloid cells, Th1 and Th2 cell differentiation (FE > 5) and B and T cell receptor signaling pathway (FE > 4) for lymphoid cells, and focal adhesion (FE > 5) and extracellular matrix-receptor interaction (FE > 4) for fibroblasts and connective tissue (Fig. 4E–G).
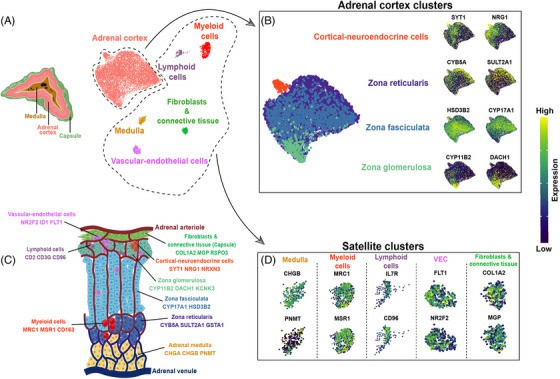 Fig. 3. Single-nucleus analysis of the adult human normal adrenal gland (Altieri B, Secener AK, et al., 2024).
Fig. 3. Single-nucleus analysis of the adult human normal adrenal gland (Altieri B, Secener AK, et al., 2024).
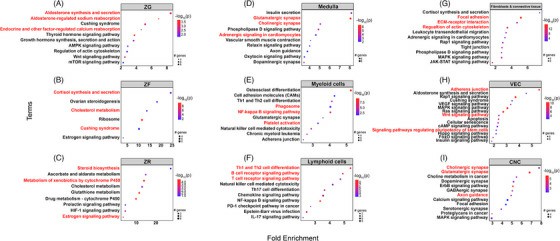 Fig. 4. Gene set enrichment analysis of single-nuclei transcriptome in adult human normal adrenal glands (Altieri B, Secener AK, et al., 2024).
Fig. 4. Gene set enrichment analysis of single-nuclei transcriptome in adult human normal adrenal glands (Altieri B, Secener AK, et al., 2024).
Ask a Question
Write your own review