Human Adrenal Cortical Cells (HAdCC)
Cat.No.: CSC-7845W
Species: Human
Source: Adrenal Gland
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The adrenal cortex, the body's main endocrine gland, maintains homeostasis by exuding steroids. It's composed of cortex and medulla, the former dominant. The cortex itself is also divided into three parts, oriented from the interior to the exterior: zona glomerulosa, zona fasciculata, and zona reticularis. Most of the cortex is composed of human adrenal cortical cells (HAdCCs). They differ in form and function from layer to layer, but are brimming with endoplasmic reticulum and mitochondria, essential for the proper production and release of steroid hormones. They are composed of glucocorticoids (such as cortisol), mineralocorticoids (such as aldosterone), and minor amounts of sex hormones. Cortisol regulates metabolism, stress and immunity; aldosterone maintains sodium and potassium, the ideal fluid and osmotic pressure; and sex hormones, although only emitted in trace quantities under normal living conditions, can become highly prevalent under pathological conditions (for example, adrenal cortical hyperplasia or tumours), where sexual nature evolves.
HAdCCs also hold substantial value in scientific research. They are employed not only in constructing models of adrenal cortical diseases, such as hyperfunction or hypofunction of the adrenal cortex, to delve into disease pathogenesis but also serve as experimental subjects in studies of steroid hormone synthesis pathways and their molecular regulatory mechanisms. Furthermore, drug screening platforms based on HAdCCs enable scientists to assess the specific impacts of drugs on hormone secretion, offering valuable tools for new drug development and efficacy evaluation.
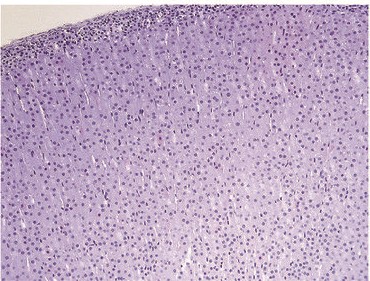 Fig. 1. Human adrenal cortical cells (Marx TK, Reddeman R, et al., 2018).
Fig. 1. Human adrenal cortical cells (Marx TK, Reddeman R, et al., 2018).
VZV-Infected qHAdCCs Produce Proinflammatory Cytokines
The viral infection of the adrenals can cause huge disturbances in the release of essential hormones, leading to symptoms such as fatigue and joint pain. Specifically, VZV causes bilateral adrenal haemorrhage and adrenal insufficiency when the virus is initially infected or after it has reactivated. But there is little to know about how VZV behaves in the adrenal glands.
Niemeyer's team examined the ability of primary human adrenal cortical cells (HAdCCs) infected with VZV to create a proinflammatory milieu. The detection of cellular proinflammatory markers indicated that IL-6 and IL-8 were higher in VZV-infected quiescence HAdCCs (qHAdCCs) than in the control supernatant, while IL-12p70, IL-13, IL-4 and TNF were only detected in the VZV supernatant (Fig. 1A;). IL-10, IL-1, IL-2, and IFN- were not present in either supernatant (Fig. 1A). No IL-10, IL-1β, IL-2, or IFN-γ were detected in either supernatant (Fig. 1A). To confirm if qHAdCCs can produce proinflammatory cytokines in response to other known adrenal cell pathogens, cells were treated with LPS. LPS-treated qHAdCCs showed a significant increase in IL-6, IL-8, IL-12p70, IL-13, IL-4, and TNF-α compared to others (Fig. 1B). IL-6 and IL-8 were especially higher in these conditions, along with increased IL-10 and IL-1β. There was no IL-2 or IFN-γ in LPS supernatants. This indicates this suggests VZV causes a proinflammatory environment during productive infection of qHAdCCs.
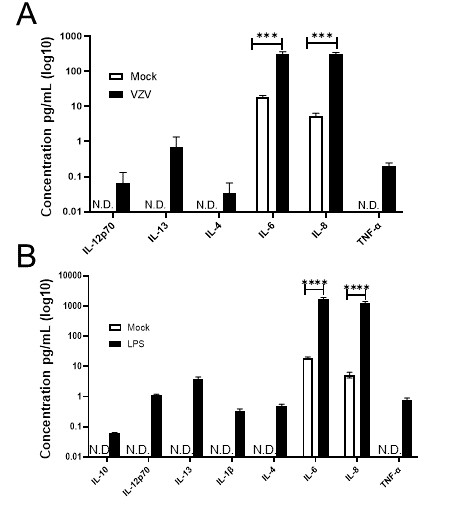 Fig. 1. VZV-infected quiescent human adrenal cortical cells (qHAdCCs) secrete proinflammatory cytokines (Niemeyer CS, Mescher T, et al., 2022).
Fig. 1. VZV-infected quiescent human adrenal cortical cells (qHAdCCs) secrete proinflammatory cytokines (Niemeyer CS, Mescher T, et al., 2022).
BEX1 Inhibits Cell Death by Ferroptosis in Adrenocortical Cells
APAs are one of the most common causes of primary aldosteronism. APAs are infected by somatic mutations in genes encoding ion channels (KCNJ5, CACNA1D, CACNA1H, CLCN2) and transporters (ATP1A1, ATP2B3). These mutations break ion homeostasis and stimulate Ca2+ signalling, resulting in increased expression of the aldosterone synthase CYP11B2, and constitutive aldosterone production. Yet it's not clear how these mutations affect cell death and proliferation.
To gain deeper insights into the tumorigenic mechanisms of APA, Yang et al. analyzed the transcriptomes of APAs of varying sizes through mRNA sequencing. They compared nine APAs with diameters ≥30 mm to twelve APAs with diameters ≤10 mm, focusing on differential genes potentially involved in cell death and proliferation. They therefore chose the gene BEX1, which codes for brain-expressed X-linked 1, as their next target. They investigated BEX1's capacity to prevent ferroptosis-induced cell death by introducing BEX1 with C-terminal DYKDDDDK (BEX1-DDK) into established human adrenocortical cell lines (Fig. 2A). Immunofluorescence staining showed BEX1-DDK's presence in the nucleus and cytoplasm (Fig. 2B). Cell cycle phases (G0/G1, S, and G2/M) were similar between HAC15 control and BEX1-DDK cells (Fig. 2C and D). In a cell viability assay, 2 μM staurosporine affected both BEX1-DDK and HAC15 control cells similarly (Fig. 2E). However, 4 μM RSL3 (a ferroptosis inducer) resulted in significantly less cell death in HAC15 BEX1-DDK cells compared to HAC15 control cells (Fig. 3A and B). Flow cytometry with propidium iodide confirmed BEX1's protective effect against RSL3-induced death (Fig. 3A, B, and D). RSL3's specificity was shown by the lack of response in the presence of 10 μM liproxstatin-1, a ferroptosis inhibitor (Fig. 3C and D). The above results show that BEX1 inhibits cell death by ferroptosis in adrenocortical cells.
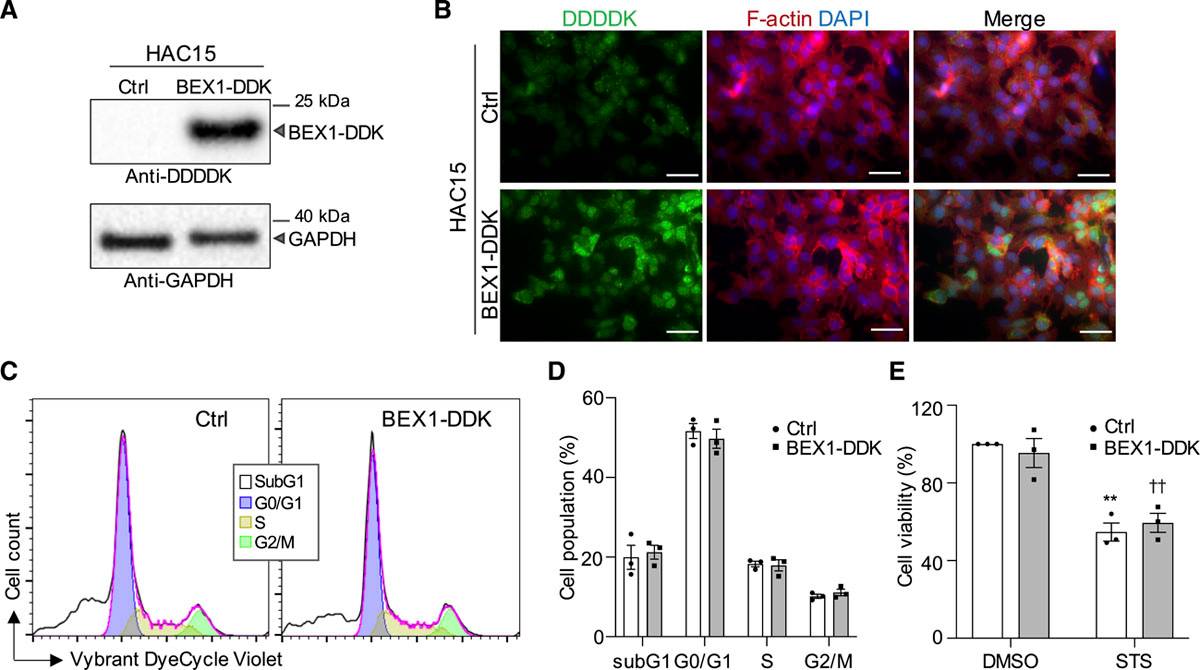 Fig. 2. BEX1 has no effect on cell cycle progression and apoptosis in adrenocortical cells (Yang Y, Tetti M, et al., 2021).
Fig. 2. BEX1 has no effect on cell cycle progression and apoptosis in adrenocortical cells (Yang Y, Tetti M, et al., 2021).
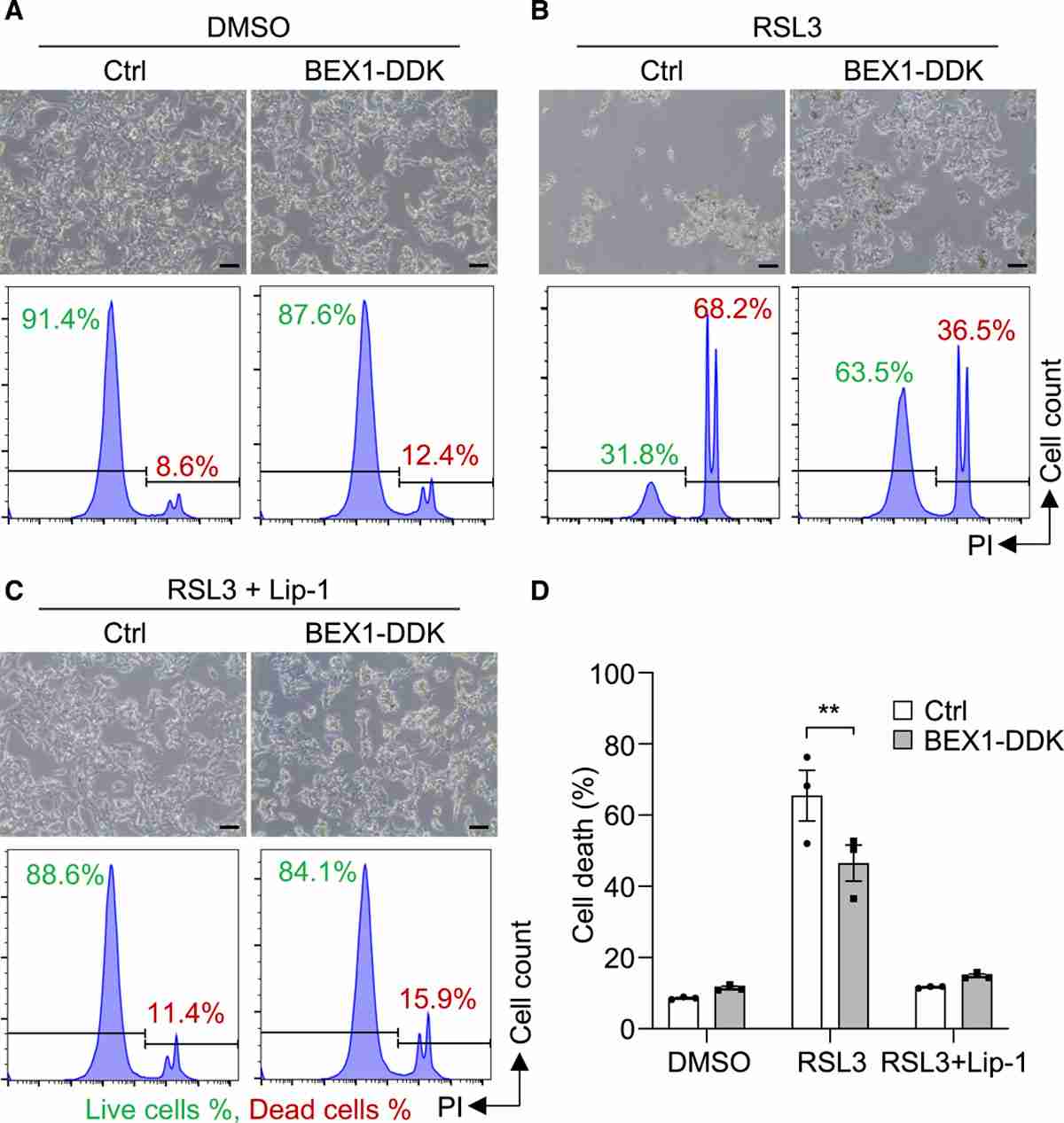 Fig. 3. BEX1 inhibits cell death by ferroptosis in adrenocortical cells (Yang Y, Tetti M, et al., 2021).
Fig. 3. BEX1 inhibits cell death by ferroptosis in adrenocortical cells (Yang Y, Tetti M, et al., 2021).
Ask a Question
Write your own review