ONLINE INQUIRY
HP2 Heps 20 Human Pooled Primary Hepatocytes
Cat.No.: CSC-C4109X
Species: Human
Source: Liver
Cell Type: Hepatocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
Each donor's hepatocytes have been cryopreserved ONCE immediately after isolation and purification.
Pools of 10 or 20 donors are available. Each donor is represented by a statistically significant number of cells relative to the pool.
There is extensive quality control providing characterization of viability and enzyme activity from both individual donors and the total pool.
Creative Bioarray guarantees performance and quality.
Donor information is available.
Creative Bioarray's HP2 Heps 20 human pooled primary hepatocytes are primary hepatocytes isolated from 20 adult liver donors. This diverse pool of cells from a range of genders, ages and diseases, eliminates potential intra-donor variability and allows for greater functional, activity and phenotypic stability. This population-based pool is, in turn, a more representative cell pool, which has far-reaching uses in drug metabolism, cytotoxicity, pathology, regenerative medicine and personalized medicine.
When assessing drug metabolism, aggregated hepatocytes better model individual variation, helping scientists understand how drugs go through the human metabolic process. They also faithfully mimic the physiological landscape of the human liver, providing a complete enzyme profile that includes the cytochrome P450 enzyme network and other drug-metabolizing enzymes, crucial for determining drug metabolic rates and pathways. Pooled hepatocytes provide a more stable, genetically diverse model of disease, emulating the complexity of the human liver. This lets scientists create disease models that mimic as closely as possible real-life conditions, essential for studying the mechanism of disease and assessing the performance of drugs. Additionally, the pooled hepatocytes also possess better regenerative potential. Since they incorporate cells from multiple donors, they are essential for understanding cell interactions and environmental influences during liver regeneration. Besides, they serve as a viable cell source for producing bioartificial livers and 3D bioprinting platforms, making them valuable resources in liver tissue engineering and regenerative medicine with massive potential for future research and applications.
 Fig. 1. Primary culture of adult hepatocytes (Joshi M G, Gadgil A, et al., 2014).
Fig. 1. Primary culture of adult hepatocytes (Joshi M G, Gadgil A, et al., 2014).
Heterocyclic Amines Disrupt Lipid Homeostasis in Cryopreserved Human Hepatocytes
Heterocyclic amines (HCAs) are mutagens generated by high-temperature meat cooking. Although HCA effects on liver lipids had already been associated with insulin resistance and type II diabetes, no one had explored how HCA affected liver lipids. Walls et al. tested the lipid homeostasis of two HCAs, MeIQx and PhIP, in cryopreserved human liver cells.
These results showed a substantial increase in the quantity of lipid droplets and neutral lipids in human hepatocytes exposed to MeIQx and PhIP (Fig. 1A-C). Expression of genes associated with lipid droplets increased patatin-like phospholipase domain-containing protein 3 (PNPLA3) and hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) dramatically in human hepatocytes following PhIP but not MeIQx treatment (Fig. 1D-E). Perilipin 2 (PLIN2) was significantly higher following PhIP and MeIQx (Fig. 1F). Neutral lipids are composed of mostly triglycerides and cholesterol esters.
Neutral lipids primarily consist of triglycerides and cholesterol esters. They then analyzed gene expression for triglycerides and associated genes. The results did not alter triglyceride content after MeIQx administration, but it did rise considerably following PhIP administration. Fatty acid synthase (FAC) and a cluster of differentiation 36 (CD36) grew rapidly only following PhIP, while stearoyl-CoA desaturase (SCD) grew rapidly only following MeIQx, and diacylglycerol O-acyltransferase 2 (DGAT2) grew rapidly both following MeIQx and PhIP. CPT1A significantly decreased following PhIP treatment (Fig. 2A-G). Total cellular cholesterol content detection showed that content the in human hepatocytes significantly increased after MeIQx and PhIP treatment, while the expression of PON1, a gene encoding a protein that stimulates cholesterol efflux, significantly decreased (Fig. 2H-I).
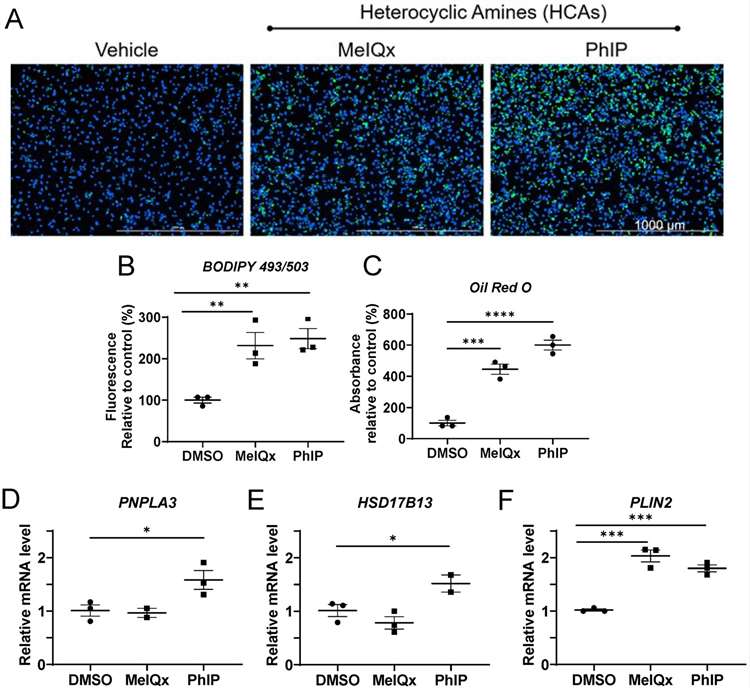 Fig. 1. Lipid droplet and neutral lipid accumulation following HCA exposure in cryopreserved human hepatocytes (Walls, K. M., Joh, J. Y., et al., 2024).
Fig. 1. Lipid droplet and neutral lipid accumulation following HCA exposure in cryopreserved human hepatocytes (Walls, K. M., Joh, J. Y., et al., 2024).
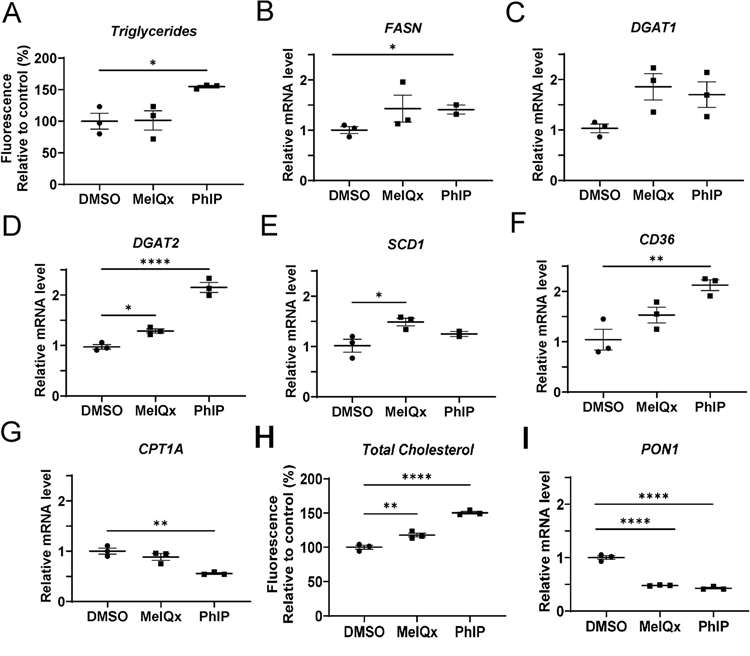 Fig. 2. Changes in intracellular triglycerides and expression of genes involved in lipid synthesis and metabolism (A-G) and cholesterol and PON1 (H-I) following HCA exposure in cryopreserved human hepatocytes (Walls, K. M., Joh, J. Y., et al., 2024).
Fig. 2. Changes in intracellular triglycerides and expression of genes involved in lipid synthesis and metabolism (A-G) and cholesterol and PON1 (H-I) following HCA exposure in cryopreserved human hepatocytes (Walls, K. M., Joh, J. Y., et al., 2024).
In Vitro Metabolism of Evodiamine in Human Liver Microsomes and Hepatocytes
Evodiamine is an indoloquinazoline alkaloid isolated from the fruit of Evodia rutaecarpa and it has anti-tumor and anti-inflammatory effects. Further purifications of its metabolites have not yet been published. Zhang et al. applied UHPLC-Q precise mass spectrometry to the metabolites of evodiamine, following its incubation with human liver microsomes and hepatocytes, to examine its metabolism in the human body.
The analysis revealed four pathways of evodiamine metabolism in human liver microsomes and hepatocytes. The first is oxidation of indole moiety to oxygenated metabolites M1 and M9, from which they are further metabolized to produce GSH conjugates (M3 and M4) through reactive quinone-imine intermediate, glucuronide conjugates (M5 and M10), and di-oxygenation metabolites (M11 and M13). The second route is C-3 position oxidation to create M2, which were further processed by oxygenation, glucuronidation and N-demethylation to create M6, M7 and M8, respectively. Third, it undergoes N-demethylation to generate M16, which was converted into oxygenated metabolites (M8, M17, M18 and M19). The fourth metabolic route is direct conjugation with GSH through reactive imine-methide intermediate to create GSH adducts (M14 and M15). Thus, oxygenation, demethylation, GSH conjugation and glucuronidation were the most common metabolic routes for evodiamine in human liver microsomes and hepatocytes.
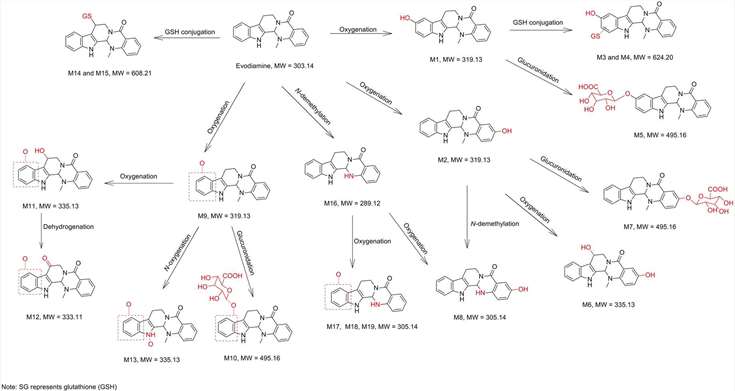 Fig. 3. Proposed metabolic pathways of evodiamine in human hepatocytes and liver microsomes (Zhang, Z., Fang, T., et al., 2018).
Fig. 3. Proposed metabolic pathways of evodiamine in human hepatocytes and liver microsomes (Zhang, Z., Fang, T., et al., 2018).
Ask a Question
Write your own review







