ONLINE INQUIRY
HP1 Heps-s Human Primary Hepatocytes-Single Donor
Cat.No.: CSC-C4110X
Species: Human
Source: Liver
Cell Type: Hepatocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
Suitable for induction assays or metabolic characterization assays in plated or suspension cultures. Plated cultures are excellent for long-term intrinsic clearance assays.
Hepatocytes are cryopreserved ONCE immediately after isolation and purification.
Characterization of viability and enzyme activity, including fold-induction of CYP1A2, CYP2B6 and CYP3A4 for HP1 Heps-i.
Creative Bioarray guarantees performance and quality.
Donor demographics and medical information are available.
The HP1 Heps-s human primary hepatocytes-single donor cell line of Creative Bioarray is a suspension cultured primary human hepatocyte cell line. Suspended human hepatocytes (SHHs) are hepatocytes that do not adhere to a substrate but multiply in culture medium as individual cells or small aggregates of cells, thereby reducing cell-to-cell aggregation and allowing for more extensive nutrition delivery. These cells are a valuable in vitro model for their unique properties and wide-ranging potential applications, and are currently being widely used in drug metabolism, toxicity studies, and disease model studies.
Unlike the traditional in vitro liver microsome model, SHHs contain a much broader group of enzymes, including enzymes belonging to the family cytochrome P450 (CYP450), which is fundamental for drug metabolism. Further, hepatocytes are the only cells that have a complete cellular machinery that can better reflect the cellular-level processes of drug diffusion, transport and metabolism. Contrasted with adherent hepatocytes, SHHs maintain CYP450 activity that most closely mimics in vivo activity during the first 4-6 hours, making them ideally suited for early metabolic stability and metabolite profiling experiments to yield more specific and accurate data. In cytotoxicity assays, the SHHs model allows researchers to compare cytotoxicity and hepatotoxicity of different drugs under more physiologically realistic circumstances, a valuable contribution to drug safety test. Additionally, because of their suspension growth, these cells can be cultured in a scalable way, which is hugely beneficial for high-throughput screening and industrial use. As a well-developed in vitro model, SHHs are not only crucial for drug metabolism, toxicity studies and disease model studies but will also likely increasingly be central to future drug discovery and biotechnology.
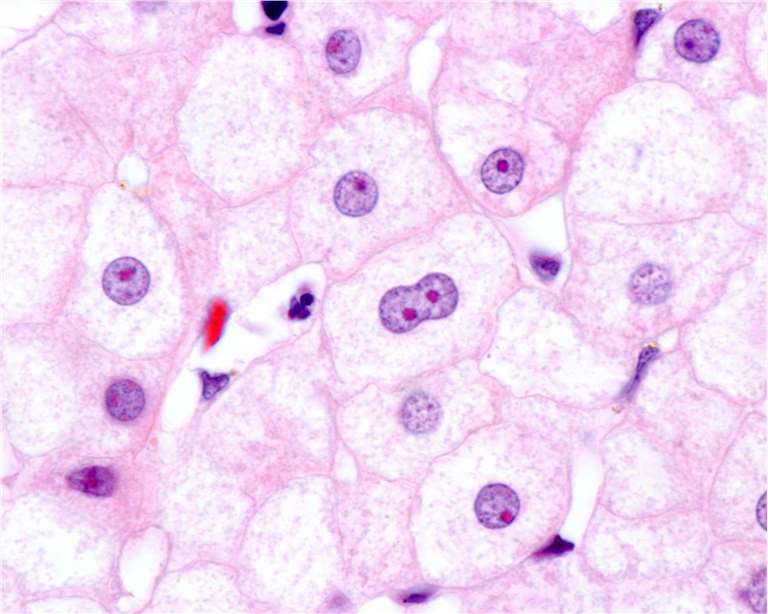 Fig. 1. Hepatocytes showing a large nucleolus stained with eosin.
Fig. 1. Hepatocytes showing a large nucleolus stained with eosin.
Comparison of Initial Uptake Clearance, C/M Ratio, and Tissue Unbound Fraction (fT) of the Six Anionic Compounds between PHH and SHH.
Among the various in vitro hepatocyte models currently established, suspended human hepatocytes (SHH) are utilized for assessing drug uptake and metabolism, while primary human hepatocytes (PHH) are used for the evaluation of drug uptake, basolateral efflux, and metabolism. These models exhibit different advantages and limitations under various experimental conditions. Robust clinical evidence indicates that organic anion transporting polypeptides (OATP) 1B1 and OATP1B3 play pivotal roles in the hepatic uptake of numerous anionic drugs. Yoshikado, et al. aims to compare the differences between PHH and SHH in determining hepatic clearance for several OATP1B substrate drugs.
They determined and compared the kinetic parameters of different batches of PHH amd SHH. Substrates including DHP and SC-62807 for OATP1B were also analyzed for rat and human liver distribution by positron-emission tomography. The PSinf (influx intrinsic clearance through the sinusoidal membrane) values for CRV, PTV, and RSV were similar between PHH and SHH, but PRV, DHP, and SC-62807 showed 5- to 10-fold lower values in PHH compared to SHH (Fig. 1A). The concentration-to-medium (C/M) ratios at 37°C for the high uptake compounds (CRV, PTV, RSV) were 3-5 times higher in PHH than SHH (Fig. 1B), whereas the low uptake compounds (PRV, DHP) exhibited roughly half the values in PHH compared to SHH. The fT (unbound fraction in hepatocytes) values were mostly comparable between both cell types (Fig. 1C). The Kp,uu (cell-to-medium unbound concentration ratio) values were higher in PHH than SHH, with fold differences as follows: 1.9 for DHP, 2.7 to 4.4 for PRV, PTV, SC-62807, and CRV, and 13 for RSV (Fig. 1D). The interlot differences assessed by the coefficient of variation (CV) were modest for hepatic uptake clearance of PTV and RSV regardless of the use of PHH and SHH. Hydrophilic compounds like SC-62807, PRV, and DHP showed low initial uptake in PHH with higher CV values than in SHH, possibly due to drug wash-off effects. Conversely, the hydrophobic CRV displayed higher CV values in SHH than PHH, likely reflecting variable non-specific adsorption to the cell surface.
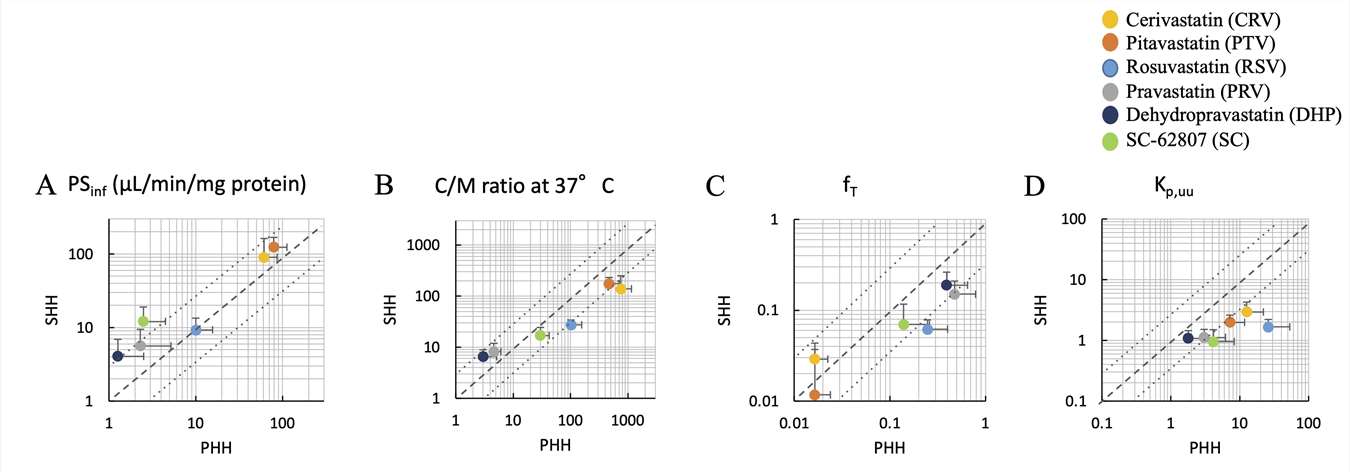 Fig. 1. Comparison of kinetic parameters for six compounds obtained using PHHs and SHHs (Yoshikado, T., Lee, W., et al., 2020).
Fig. 1. Comparison of kinetic parameters for six compounds obtained using PHHs and SHHs (Yoshikado, T., Lee, W., et al., 2020).
A Novel Unified Approach to Predict Human Hepatic Clearance for Both Enzyme- and Transporter-Mediated Mechanisms Using Suspended Human Hepatocytes
Riccardi, et al. introduce a novel method using suspended human hepatocytes to predict human hepatic clearance for compounds, which may be substrates of drug-metabolizing enzymes and/or transporters.
Riccardi, et al. examine 32 structurally diverse compounds, covering five ECCS classes (1a, 1b, 2, 3b, 4). It summarizes the physicochemical and in vitro data for compounds unlikely to have biliary excretion (ECCS 1a, 1b, and 2) and those with potential significant biliary excretion (ECCS 3b and 4). For compounds without significant biliary excretion (19 compounds), the novel extended clearance method (Method I) showed good IVIVE results, with most compounds (16 out of 19) having predicted values within threefold of observed values. Outliers included pitavastatin, telmisartan, and theophylline. Conversely, the traditional method (Method II), which considers only metabolic clearance, systematically underpredicted hepatic clearance, with only 26% of predictions within threefold of observed values, indicating that transporter-mediated clearance is also important. Similar trends were observed in predicting hepatic intrinsic clearance. The AFE for hepatic clearance using Method I and II were 1.9 and 4.1, respectively, and the bias values were 0.84 and 0.30, indicating Method I's higher accuracy (Fig. 2). For compounds with potential significant biliary excretion (13 compounds), 38% of predictions using Method I were within threefold, compared to 23% using Method II. Intrinsic clearance was also underpredicted (Fig. 3). AFE and bias values confirmed that Method I is more reliable for these compounds.
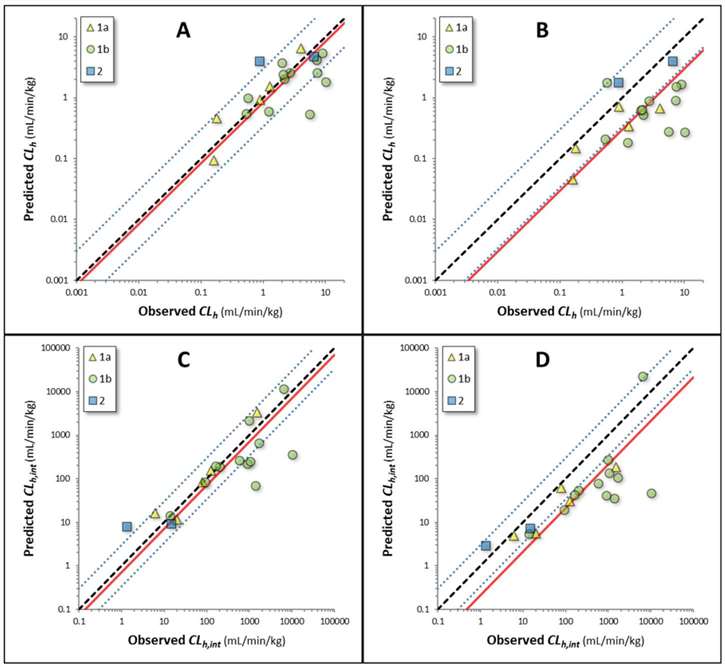 Fig. 2. Observed and predicted clearance for compounds without the potential for biliary excretion as a major clearance pathway (Riccardi, K. A., Tess, D. A., et al., 2019).
Fig. 2. Observed and predicted clearance for compounds without the potential for biliary excretion as a major clearance pathway (Riccardi, K. A., Tess, D. A., et al., 2019).
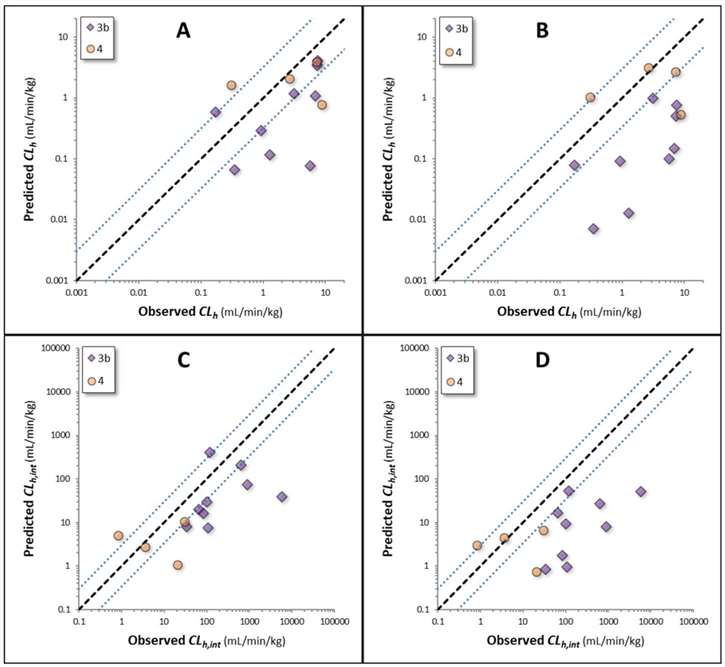 Fig. 3. Observed and predicted clearances for compounds with the potential for biliary excretion as a major clearance pathway (Riccardi, K. A., Tess, D. A., et al., 2019).
Fig. 3. Observed and predicted clearances for compounds with the potential for biliary excretion as a major clearance pathway (Riccardi, K. A., Tess, D. A., et al., 2019).
Ask a Question
Write your own review







