ONLINE INQUIRY
Fetal Liver Cells CD34+ Cells
Cat.No.: CSC-C4502X
Species: Human
Source: Liver
Cell Type: CD34+ Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Fetal liver-derived CD34+ cells are a major component of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HSPCs). CD34 is a highly glycosylated type I transmembrane glycoprotein that's expressed only on the surface of human and other mammalian HSCs and HSPCs, and its activity decreases with cell maturation. Cells displaying the CD34 antigen can be isolated and purified using immunomagnetic separation (MACS). Among the CD34+ cells in the fetal liver, which can differentiate into a broad range of blood cells such as erythrocytes, leukocytes and thrombocytes to maintain normal hematopoiesis, are central to hematopoietic function. What's more, CD34+ cells play a key role in the immune system regulation by mediating cell adhesion, in inflammatory activity, and in lymphocyte homing to regulate immune homeostasis.
As the fetal grows, the amount of CD34+ cells in the fetal liver fluctuates. Notably, during mid-gestation (11 to 20 weeks), the fetal liver contains relatively high numbers of CD34+ cells. This temporal peak enables the harvesting of large numbers of HSCs and progenitor cells that, with their high proliferative and differentiation potential during this gestational period, are unbeatable resources for research and therapeutic use. Fetal liver CD34+ cells have superior proliferative capabilities when compared with adult bone marrow and umbilical cord blood cells because they are longer in telomeres and lack human leukocyte antigen (HLA) expression. They therefore have great therapeutic potential for hematopoietic stem cell transplantation, gene therapy, regenerative medicine and immunotherapy.
GH Supplementation Hastens Human Lymphohematopoietic Cell Recovery in the Blood
Humanized mice generated through human hematopoietic stem cell (HSC) transplantation play a critical role in biomedical research. One of the factors hindering the efficacy of their construction is the lack or insufficiency of interactions between human cells and murine cytokines and growth hormones (GH). GH is a pluripotent cytokine that can regulate the proliferation, apoptosis, and differentiation of various cells. In this study, Zhang's team investigated the effects of recombinant human growth hormone (rhGH) on humanized mice transplanted with CD34+ cells.
Humanized mice were generated by transplanting human CD34 fetal liver cells from immunodeficient mice pretreated with total body irradiation. Subsequently, peripheral blood, spleen and bone marrow were harvested, and levels of human lymphohematopoietic cells were determined by flow cytometry. The low amino acid identity between mouse GH (mGH) and human GH (hGH) suggests that mGH may not provide survival or proliferation signals to the human GH receptor (hGHR) on the human cells engrafted in mice. Therefore, they investigated whether supplementation with a hGHR agonist could optimize the establishment of a human lymphohematopoietic system. They treated NCG mice with transplanted human CD34 fetal liver cells with hGH. Human lymphohematopoietic chimerism in the hGH-treated mice was significantly higher (Fig. 1). Human B cells and myeloid cells are the major human lymphohematopoietic cell populations in NCG mice having undergone human CD34 cell transplantation at adult stages. Therefore, they also analyzed the recovery of human CD19 B cells and CD33 myeloid cells in the blood of these mice. Similarly, the recovery of both human B cells and myeloid cells was significantly enhanced by hGH treatment (Fig. 1).
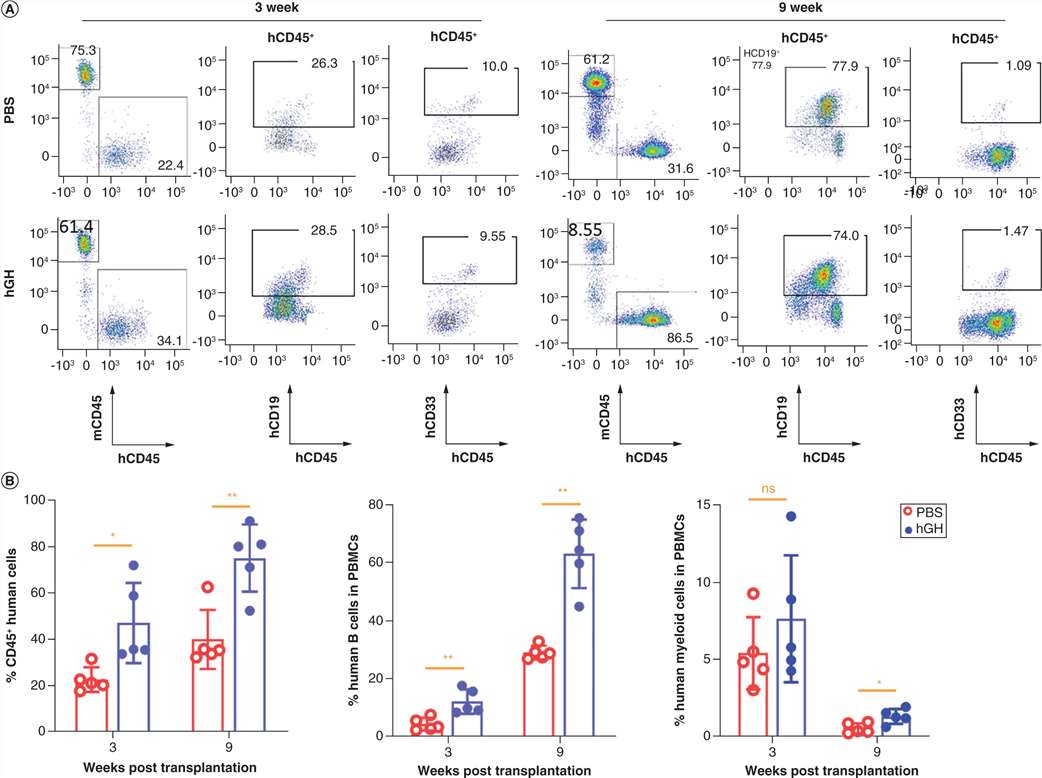 Fig. 1. Growth hormone supplement hastens human lymphohematopoietic cell reconstitution in the blood of NCG mice (Zhang, S., Wang, G., et al., 2022).
Fig. 1. Growth hormone supplement hastens human lymphohematopoietic cell reconstitution in the blood of NCG mice (Zhang, S., Wang, G., et al., 2022).
SRSF2 P95H Mutation Reduces Colony Formation and Skews Towards Monocytic Lineage in Human CD34+ Fetal Liver Cells
SRSF2 is a splicing factor that normally plays a critical role in the precursor RNA splicing process. However, mutations in SRSF2 disrupt this process, leading to aberrant alternative splicing events. These abnormal splicing events can activate a global gene expression program, which drives the abnormal proliferation and transformation of hematopoietic cells into malignant cells. Using high-throughput sequencing of RNA isolated by UV crosslinking and immunoprecipitation (HITS-CLIP), Liang's team found SRSF2 P95H mutations impact RNA binding and splicing, especially in RNA processing and splicing genes, suggesting a "splicing-cascade" effect. This cascade may lead to widespread gene alterations and impaired hematopoietic differentiation, ultimately driving cancer.
To characterize the in vivo effects of SRSF2 P95H mutation in the hematopoietic context, Liang's team generated stable, isogenic Human Erythroid Leukemia (HEL) cell lines with lentivirally conferred inducible expression of Flag-tagged wild-type (WT) and mutant (P95H) SRSF2 (Fig. 2a). And then, Liang's team tested their lentiviral construct in primary human CD34+ fetal liver cells, and performed Colony Forming Unit (CFU) assays to monitor the impact of SRSF2 mutations on hematopoiesis. The total number of colonies was significantly reduced upon SRSF2P95H expression compared to WT SRSF2, with a relative increase of monocytic lineage colonies (CFU-M) in colony composition (Fig. 2c), consistent with the monocytic lineage skewing observed in previous studies and the preferential occurrence of SRSF2 mutations in chronic myelomonocytic leukemia (CMML).
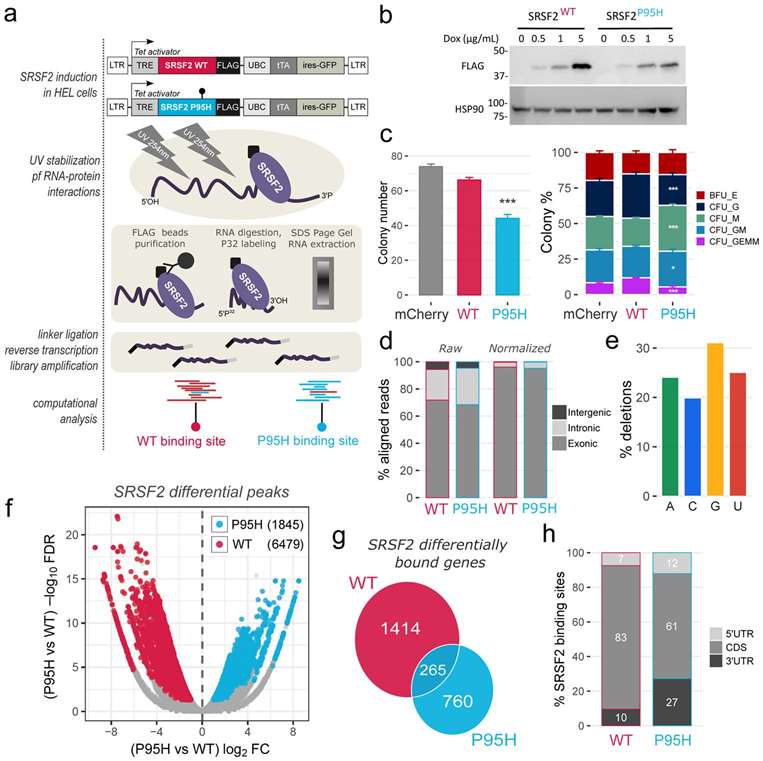 Fig. 2. The SRSF2 P95H mutation alters SRSF2 in vivo RNA interactome (Liang, Y., Tebaldi, T., et al., 2018).
Fig. 2. The SRSF2 P95H mutation alters SRSF2 in vivo RNA interactome (Liang, Y., Tebaldi, T., et al., 2018).
Ask a Question
Write your own review