ONLINE INQUIRY
Rat Oligodendrocyte Precursor Cells
Cat.No.: CSC-C9521J
Species: Rat
Source: Brain
Cell Type: Oligodendrocyte Progenitor Cell; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
ROPC are isolated from postnatal day 2 CD(SD) IGS Rat brain. ROPC are cryopreserved after purification and delivered frozen. ROPC are characterized by immunofluorescence with antibodies specific to A2B5 and O1. ROPC are negative for mycoplasma, bacteria, yeast, and fungi. ROPC are guaranteed to further culture under the conditions provided by Creative Bioarray; however, ROPC are not recommended for expanding or long-term cultures due to limited expansion capacity.
SuperCult® Oligodendrocyte Precursor Cell Differentiation Medium for differentiating Rat Oligodendrocyte Precursor Cells.
Note: Never can cells be kept at -20 °C.
Oligodendrocyte precursor cells (OPCs) are tissue-resident stem cells that populate all regions of the central nervous system (CNS) and persist after development. Differentiation of OPCs into myelination-capable mature oligodendrocytes is essential for normal function of the CNS. In addition to their progenitor role, OPCs have been proposed to play other functions, including modulating the development of axons and synapses and participating in bidirectional signaling with neurons and other glial cells.
Biological Functions of OPCs
The canonical role of OPCs is to give rise to myelinating oligodendrocytes. However, additional functions have been suggested for a long time. For example, fine-tuning the size of neural circuits and axonal arbors may be through phagocytosis and pruning of axons. OPCs also have immunomodulatory capabilities, as they express cytokine receptors and can cross-present antigens to cytotoxic CD8+ T cells. Furthermore, OPCs have synaptic signaling properties due to their connections to neurons. OPCs also synapse with glutamatergic neurons in the gray and white matter. Transgenic mouse models have shown that the pattern of neuronal activity in the brain region affects glutamate release and the differentiation of OPCs. This may explain the differences in the cell cycle of OPCs in white matter and gray matter, with higher rates of proliferation and differentiation in white matter than in gray matter.
Several protocols have been developed to isolate and culture OPCs from the rodent brain at different stages from embryonic to adulthood. Primary OPCs cultures derived from rodents are particularly interesting for studying the proliferation and differentiation of OPCs and are suitable for high-throughput screening of pharmacological compounds that may interfere with these processes.
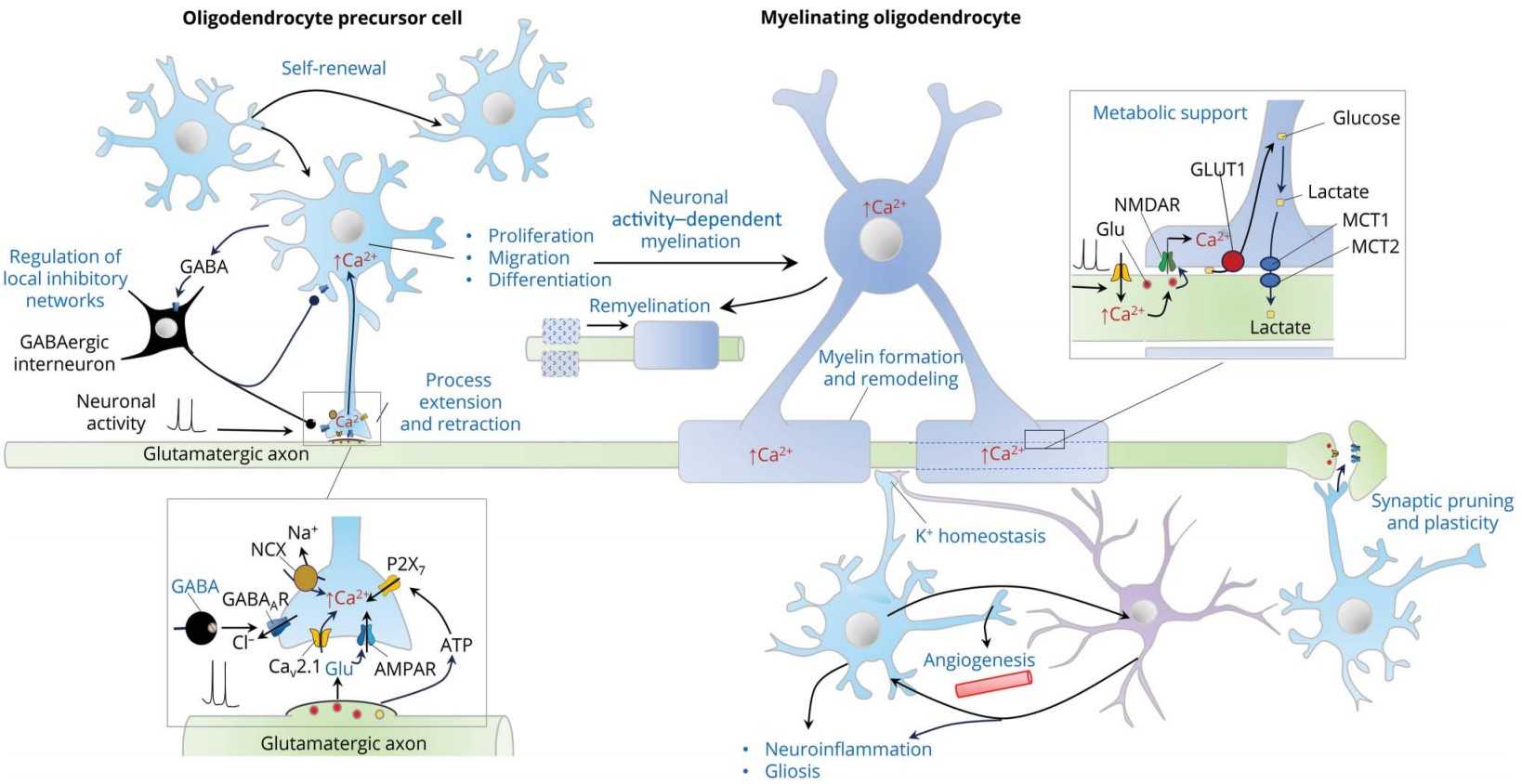 Fig. 1. Functions of oligodendrocyte precursor cells (OPCs) (Benarroch, Eduardo. 2023).
Fig. 1. Functions of oligodendrocyte precursor cells (OPCs) (Benarroch, Eduardo. 2023).
OPCs Differentiation Were Different Between Brain and Spinal Cord
Primary OPCs cultures were obtained from postnatal day 1-2 Sprague Dawley rat cerebral cortices and spinal cord. OPCs with >95% purity were identified by Calcein AM staining and the expression of oligodendrocyte lineage cell markers NG2 and MBP (Fig. 1A, B). On the third day of primary cell culture in the medium of DMEM-F12 containing 10% FBS and 1% penicillin/streptomycin, we found brain-derived cells were mainly OPCs with a dark cell body, tadpole-like, and only 2-3 branches (Fig. 1C). In contrast, spinal cord-derived OPCs were easier to differentiate than brain-derived, reflected by the significant increase in branches and processes (Fig. 1D). Immunofluorescence staining of NG2, MBP, and GFAP was further used to detect dynamic changes of OPCs in both differentiation and morphology (Fig. 1C, D). When compared with brain, we found that spinal cord-derived oligodendrocytes had larger cell areas and longer diameters after maturation (Fig. 1C-F). Spinal cord-derived oligodendrocytes had showed longer diameter and larger cell area at the 4th day, peaked at the 6th day and were maintained until the 8th day (Fig. 1E, F). Thus, there were significant differences in OPCs differentiation and oligodendrocytes morphology between the brain and spinal cord.
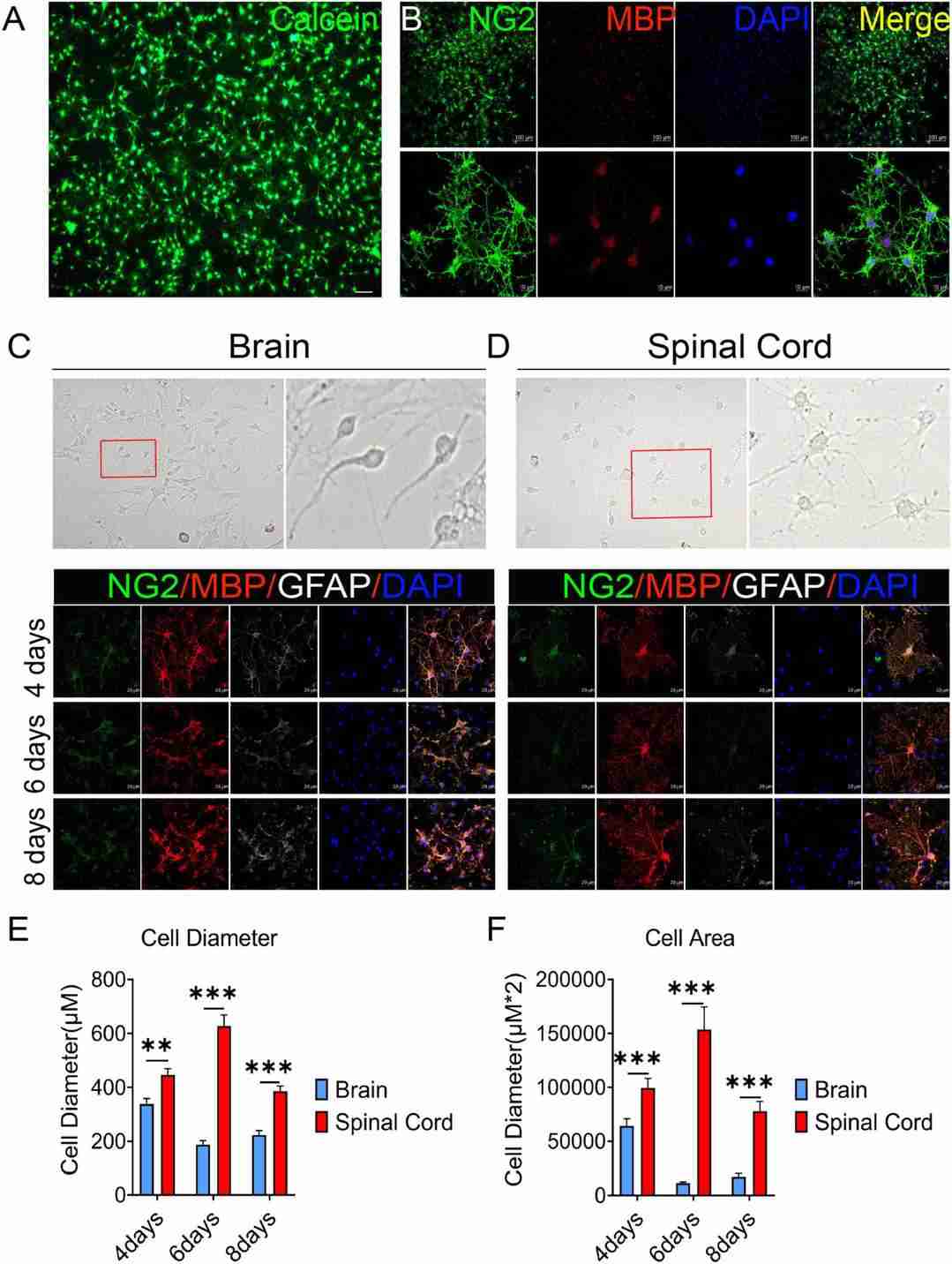 Fig. 1. The OPCs differentiation and oligodendrocytes morphology are different between the brain and spinal cord (Zhao, Qing, et al. 2022).
Fig. 1. The OPCs differentiation and oligodendrocytes morphology are different between the brain and spinal cord (Zhao, Qing, et al. 2022).
Media Composition and Growth Factors for OPC Proliferation
Isolated OPCs were proliferated in an OPC proliferation medium under varying conditions (DMEM, DMEM + EGF, DMEM/F12, DMEM/F12+EGF, Neurobasal, Neurobasal + EGF) for 5-6 days, with a full medium change on day 3 of proliferation. While immunostaining for the proliferation markers EdU and Ki67 showed no difference in the proportion of cells proliferating between media conditions, WST-8 proliferation/viability assays revealed that at both the 4- and 5-day time points, condition 4 (DMEM/F12 with EGF) showed an approximately 10-15-fold higher viable cell number than the Neurobasal-based formulae (Fig. 2a, b, c and d). Therefore, condition 4 (DMEM/F12 with EGF) was selected as the optimal OPC proliferation medium.
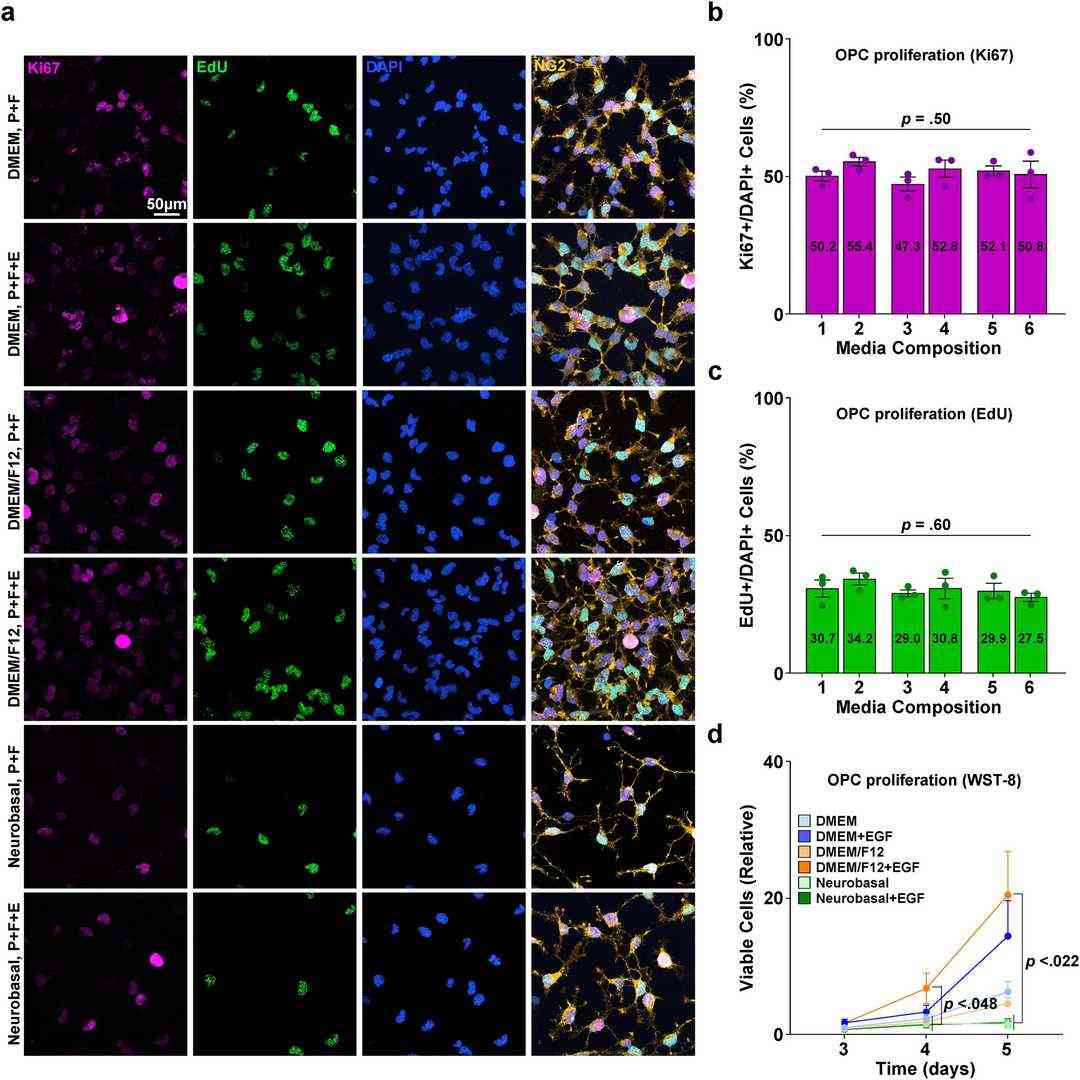 Fig. 2. Proliferation of isolated primary OPC enriched culture (Kim, Hanki, et al. 2024). P: PDGF, F: FGF, E: EGF.
Fig. 2. Proliferation of isolated primary OPC enriched culture (Kim, Hanki, et al. 2024). P: PDGF, F: FGF, E: EGF.
Ask a Question
Write your own review
- You May Also Need