ONLINE INQUIRY
Mouse Trigeminal Neurons
Cat.No.: CSC-C5350S
Species: Mouse
Source: Brain
Cell Type: Neuron
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse trigeminal neurons from Creative Bioarray are isolated from the mouse trigeminal nerve tissue. The method we use to isolate mouse trigeminal neurons was developed based on a combination of established and our proprietary methods. The mouse trigeminal neurons are characterized by immunofluorescence with antibodies specific to neuron specific enolase (NSE). Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Mouse trigeminal neurons develop from the trigeminal ganglion inside the skull where three sensory nuclei (trigeminal principal nucleus, main sensory nucleus, spinal nucleus) and a motor nucleus (trigeminal motor nucleus) are found. Sensory information from the face, head, and oral cavity reaches the central nervous system through transmission by trigeminal neurons along the fifth cranial nerve. The structure of trigeminal neurons includes standard neuronal components such as dendrites, axons and cell bodies. The cell bodies of trigeminal neurons live in the trigeminal ganglion while their axons reach into the central nervous system thereby creating the trigeminal nerve. These neurons demonstrate strong angle modulation properties under culture conditions which indicates their directional selectivity when stimulated from various directions.
Trigeminal neurons serve as messengers that relay sensory data from the facial region and oral cavity to process touch sensations and detect pain and temperature changes. These neurons serve vital functions in detecting pain and temperature changes which is highlighted by the VR1 receptor's role in pain communication. These neurons also function in autonomic nervous system regulation through mechanisms like calcium-activated chloride channels (CaV3.2) which influence chronic pain responses. Therefore, trigeminal neurons hold great importance for researchers studying pain. For instance, using CRISPR editing technology to mark mouse trigeminal neurons enables researchers to track gene expression changes after nerve injury and their effects on chronic pain.
Mouse Trigeminal Ganglion Cells Express Several Integrins Including Β4 Integrin, L1CAM, and LN332 and Preferentially Adhere to the LN332 Secreted by HCLE Cells
Intraepithelial corneal nerves are vital for corneal health, while Rho kinase inhibitors (RIs) are known for aiding neuron survival post-injury. However, their role in affecting neurite outgrowth and nerve reinnervation is less understood.
Karpinski et al. developed a coculture model using mouse trigeminal ganglion cells and HCLE human corneal epithelial cells to study neuron-corneal epithelial cell interactions. Trigeminal ganglion cells grew in neurobasal media, which also supported HCLE cell growth. We cocultured primary TG neurons with HCLE cells on FN/CNI-coated glass coverslips. Both corneal and epidermal epithelial cells adhere mainly using α6β4 and α3β1 integrins, depositing a LN332-rich extracellular matrix. As cells migrate, they leave integrin-containing footprints. Shown in Figure 1A is a representative image of neuron: HCLE cocultures stained on the left for α6 integrin in green, βIII tubulin in red to reveal axons, and DAPI to show nuclei. Asterisks show two axons and the two # symbols highlight α6 integrin+ footprints. In Figure 1B, cocultures stained to localize α6 integrin and LN332 are presented. Footprints are positive for both α6 integrin and LN332. Note that axons seem to attach preferentially to surfaces at sites where HCLE footprints are located. Neurons are indicated by white asterisk and neuroprogenitor cells indicated by orange asterisks. Neurons express abundant levels of both α6 integrin and LN332 and adhere to sites on coverslips where LN332 matrix localizes.
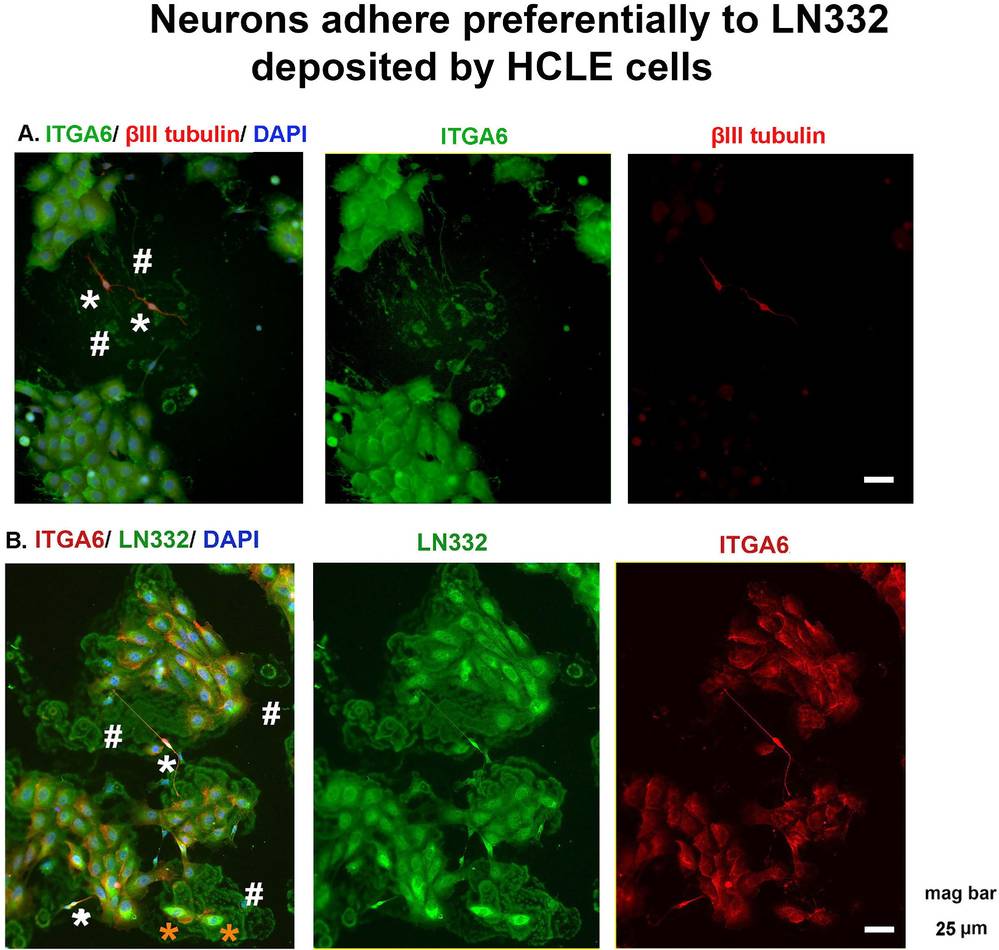 Fig. 1. Neurons adhere preferentially to LN332 deposited by HCLE cells (Yeh S, Yu S, et al., 2021).
Fig. 1. Neurons adhere preferentially to LN332 deposited by HCLE cells (Yeh S, Yu S, et al., 2021).
Excitability of Trigeminal Ganglion Neurons is Controlled by Anxa10
Trigeminal neuropathic pain (TNP) is a chronic orofacial pain syndrome characterized by spontaneous, electric shock-like facial pain and is notoriously difficult to treat. Annexins, particularly Anxa10, have been implicated in neuropathic pain processes.
Neuronal hyperexcitability in the trigeminal ganglion (TG) is linked to neuropathic pain. Miao's team investigated Anxa10's role in this excitability post-pIONL using whole-cell patch-clamp recordings on trigeminal neurons from naive and lentivirus-infected mice. Figure 2a-b shows typical action potential traces triggered by 2× rheobase currents in TG neurons, which increased after pIONL but decreased with LV-Anxa10-shRNA infection (P <0.001, Sham vs pIONL; P <0.05, pIONL +shRNA-NC vs pIONL +shRNA-1). Post-pIONL, ipsilateral neurons had lower rheobase, indicating hyperexcitability. Figure 2c shows significant shifts 7 days after pIONL. The neuron firing frequency increased post-pIONL but was reduced by Anxa10 knockdown (P <0.01, Fig2.d-e). Membrane properties remained similar across groups (Fig2.f-g). Anxa10's involvement in enhanced excitability post-pIONL is suggested.
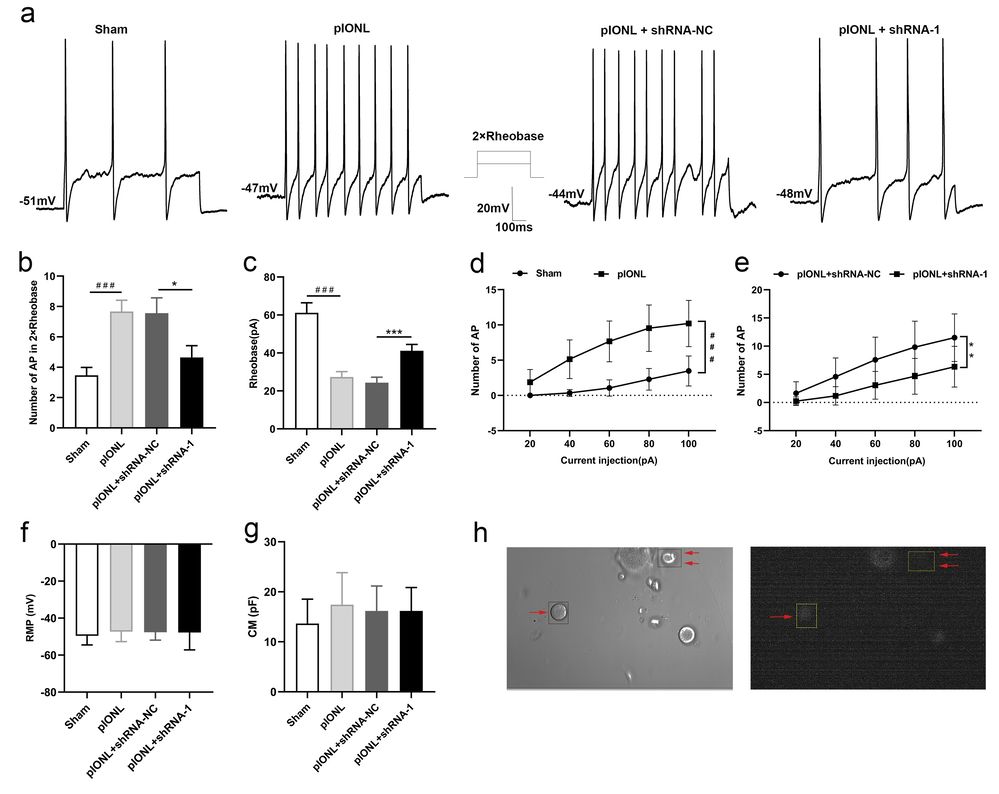 Fig. 2. Knock-down of Anxa10 reduced trigeminal ganglion (TG) neuronal hyperexcitability in mice after pIONL (Miao H, Jiang Y, et al., 2022).
Fig. 2. Knock-down of Anxa10 reduced trigeminal ganglion (TG) neuronal hyperexcitability in mice after pIONL (Miao H, Jiang Y, et al., 2022).
Ask a Question
Write your own review