ONLINE INQUIRY
Mouse Endometrial Stromal Cells
Cat.No.: CSC-C5328S
Species: Mouse
Source: Endometrium; Uterus
Cell Type: Stromal Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse endometrial stromal cells from Creative Bioarray are isolated from the mouse uterine tissue. The method we use to isolate mouse endometrial stromal cells was developed based on a combination of established and our proprietary methods. The mouse endometrial stromal cells are characterized by immunofluorescence with antibodies specific to vimentin. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
Mouse endometrial stromal cells (MESCs) derive from the stromal layer of the endometrium which forms the connective tissue underneath the endometrial epithelial cells. The endometrium consists of a mucosal lining in the uterine cavity which functions as a vital component in reproductive procedures including the implantation of embryos and the regulation of menstrual cycles.
MESCs play crucial roles in endometrial cyclic changes throughout both menstrual cycles and pregnancy periods. Various growth factors and hormones including gonadotropin-releasing hormone, tissue factor, and plasminogen activator which these cells secrete control endometrial proliferation as well as remodeling and breakdown. During embryo implantation, MESCs undergo rapid differentiation to become decidual cells which represents a major transformation within the endometrium. MESCs hold substantial significance in reproductive physiology research. For example, researchers utilize these cells to investigate the development of diseases including endometriosis and endometrial cancer and to understand infertility issues. Additionally, the use of MESCs extends to examining the processes behind endometrial regeneration and repair which includes healing endometrial injuries.
 Fig. 1. Representative picture of MESC (magnification 20X, scale bar: 100 µm) (Clercq KD, Hennes A, et al., 2017).
Fig. 1. Representative picture of MESC (magnification 20X, scale bar: 100 µm) (Clercq KD, Hennes A, et al., 2017).
Usp22 Upregulation In Vitro under Artificial Decidualization
Decidualization is essential for successful embryo implantation, with limited understanding of its molecular mechanisms. Usp22 is a deubiquitinating enzyme with roles in tumor growth, embryogenesis, and transcription regulation. Wang's team explored the role of Usp22 in uterine stromal decidualization in mice.
To investigate Usp22 dynamics during in vitro decidualization, primary mouse endometrial stromal cells (mESCs) were isolated and confirmed via positive vimentin and negative cytokeratin staining. Usp22 mainly localized in the nucleus and was weakly expressed in the cytoplasm of decidual stromal cells (Fig. 1A). Cells treated with E2 and P4 to induce decidualization showed increased Dtprp mRNA and Usp22 protein levels (Fig. 1B, C), indicating time-dependent Usp22 enhancement. Since E2 and P4 exerted minimal impact on Usp22 expression in ovariectomized mice, they further performed in vitro E2/P4 treatments for primary mESC isolated from day 4 of pregnancy. In vitro trials with primary mESCs from day 4 of pregnancy revealed that E2 had no effect on Usp22 after 24h, but P4 significantly increased its expression, which was blocked by the progesterone receptor antagonist RU486, suggesting P4 as the main regulator (Fig. 1D, E).
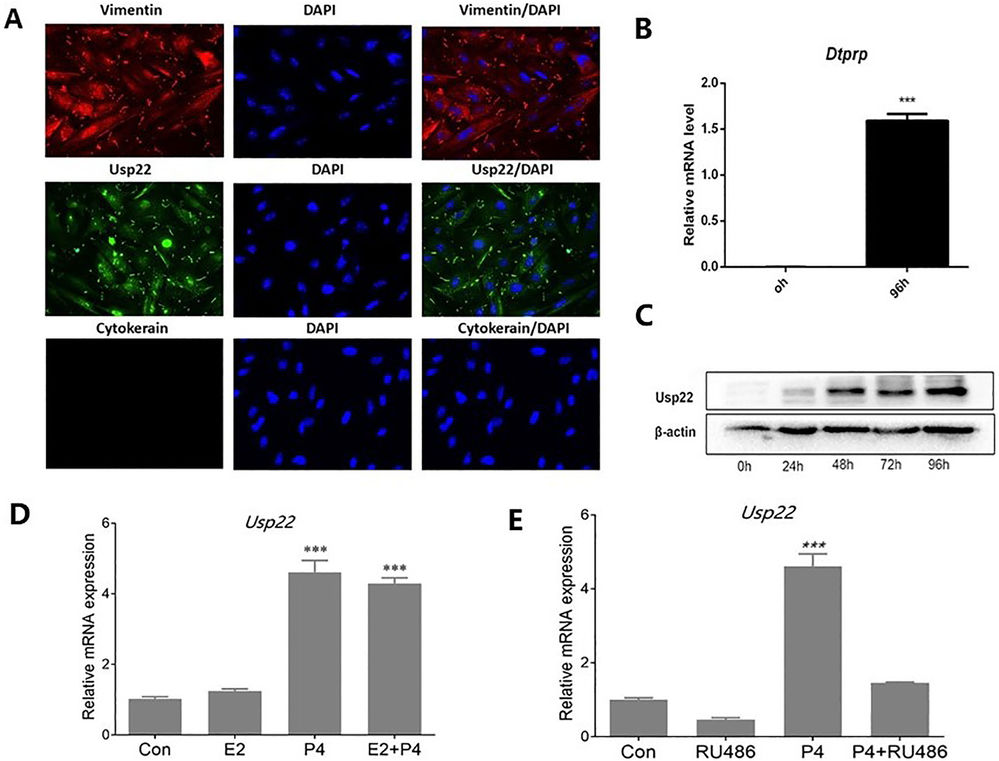 Fig. 1. Usp22 expression in the context of in vitro decidualization (Wang Y, Gao Y, et al., 2021).
Fig. 1. Usp22 expression in the context of in vitro decidualization (Wang Y, Gao Y, et al., 2021).
Isolation of ESCs and Induction of In Vitro Decidualization
Mouse endometrial stromal cells were isolated following a slightly modified published protoco. Non-pregnant CBA/J female mice were induced into estrus by subcutaneously injecting 100 µL of 17β-estradiol (100 ng/100 µL) daily for three days. The estrus phase was confirmed through vaginal smear and uterine horn histology. Vaginal cytology showed predominantly superficial epithelial cells (Fig. 2A and B), and histology indicated stromal cell proliferation and uterine gland growth (Fig. 2C). ESCs were isolated from uterine horns and cultured (Fig. 2D). Immunofluorescence staining confirmed stromal cells expressed vimentin but not cytokeratin, with 95% purity. hAECs were used as a positive control for cytokeratin staining (Fig. 2E). ESCs were in-vitro decidualized using cAMP and MPA over 6 days, changing from elongated to rounded cells with larger nuclei (Fig. 2F). Decidualization was confirmed by increased Prl and Pgr mRNA levels in treated ESCs compared to controls (Fig. 2G).
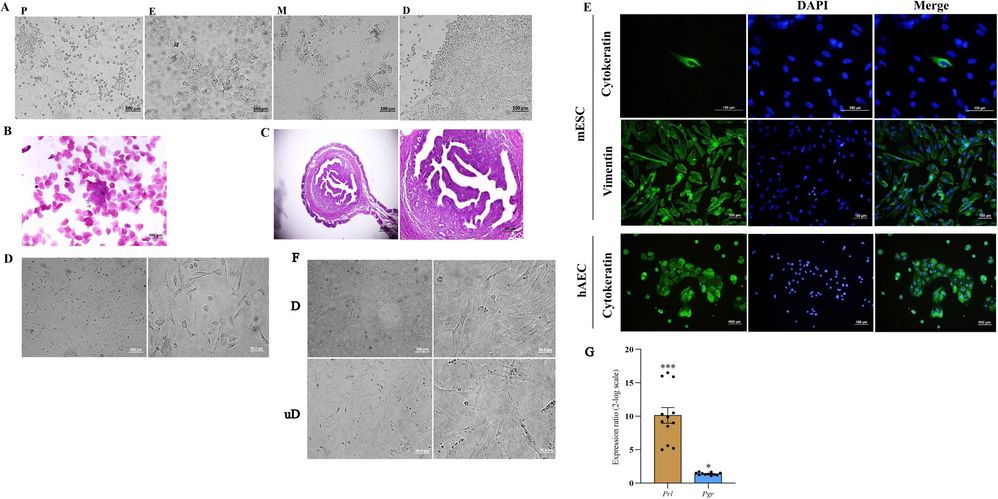 Fig. 2. Isolation of mouse endometrial stromal cells, characterization, and in-vitro decidualization (Zarnani K, Zarnani K, et al., 2024).
Fig. 2. Isolation of mouse endometrial stromal cells, characterization, and in-vitro decidualization (Zarnani K, Zarnani K, et al., 2024).
Ask a Question
Write your own review