ONLINE INQUIRY

Human Uterine Microvascular Endothelial Cells (HUMECs)
Cat.No.: CSC-C4013X
Species: Human
Source: Uterus
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Uterine Microvascular Endothelial Cells (HUMECs) from Creative Bioarray are isolated from the uterus tissue of healthy donors. The cells are cryopreserved at Passage 2 and delivered frozen. Each vial contains at least 0.5 *10^6 cells. The cells are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HUMECs are guaranteed for at least 15 population doublings under the conditions provided by Creative Bioarray. Repeated freezing and thawing of cells is not recommended.
Human Uterine Microvascular Endothelial Cells (HUMECs) are endothelial cells isolated from the human endometrium. The uterus, as an important component of the female reproductive system, is composed of the endometrium, myometrium, and serosa, with micro-vessels distributed throughout these tissue layers. In physiological conditions, HUMECs can participate in the angiogenesis process of uterine tissue, providing the necessary vascular support for the periodic repair of the endometrium and embryo implantation. At the same time, as a barrier between blood and uterine tissue, HUMECs can also provide the necessary nutrients and oxygen to the uterus, and help remove waste and carbon dioxide.
It is worth noting that, unlike human umbilical vein endothelial cells (HUVECs) commonly used in cardiovascular research, HUMECs are situated in an environment that is closer to the maternal vascular system. Although research on HUMECs is relatively limited at present, they hold potential application value in the field of endometrial disease research, such as in the study of endometriosis and endometrial cancer. In addition, given the important role of endometrial cells during pregnancy, HUMECs may also provide a valuable model for studying pregnancy-related physiological changes and pathological conditions. In the field of regenerative medicine, by inducing the proliferation and differentiation of HUMECs, it is possible to generate vascular tissues with specific functions.
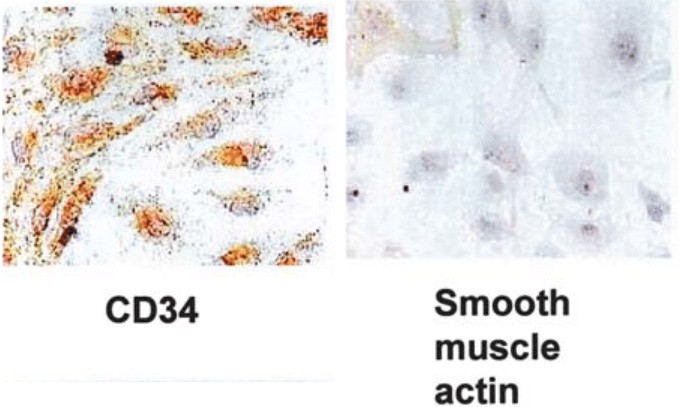 Fig. 1. Characterization of cultured human uterine microvascular endothelial cells (Kitaya K, Yasuo T, et al., 2007).
Fig. 1. Characterization of cultured human uterine microvascular endothelial cells (Kitaya K, Yasuo T, et al., 2007).
Calcium Deficient Placental Growth Restriction is Mediated by an Increase in Non-Invasive Integrin α5 and β4 Phenotype
Integrins are crucial in trophoblast invasion during early placental development, a process implicated in preeclampsia—a major pregnancy complication. Preeclampsia, featuring endothelial dysfunction, poses significant maternal risks, particularly when prevention is challenging. Nutritional interventions, like calcium supplementation, offer potential preventive measures. Xu's team explored how calcium levels affect cellular pathways and integrin switching, impacting trophoblast integration into endothelial networks in vitro.
A cell model using red-labeled endothelial and green-labeled HTR-8/SVneo trophoblast cells was co-cultured with varying calcium concentrations to observe effects on cell integration. Gene expression and cytokine concentrations were analyzed using PCR, ELISA, and Luminex assays. As shown in Fig. 1a, trophoblast cell integration into endothelial networks was reduced in low calcium (1.8 mM, −11 ± 2%) and calcium depletion groups (0.4 mM, −24 ± 3%) compared to the normal calcium group (2.4 mM, set as 100%). IL-6 levels in culture media were also lower in low calcium (−14 ± 4%) and calcium depletion groups (−18 ± 3%) than in normal calcium (Fig. 1b). No significant differences were observed in other cytokines, invasion markers, or angiogenic markers among the groups. In the calcium depletion group, mRNA for integrins α5 and β4 increased (Fig. 2a, b), but integrin α1 decreased (Fig. 2a), with no differences in integrins α6 (Fig. 2c) and β1 (Fig. 3) expression across groups. To sum up, the in vitro trophoblast cell integration into endothelial cellular networks could be modified by altering media calcium through integrin switch away from integrins α5 and β4 and towards integrin α1 which may be required for healthy early trophoblast integration.
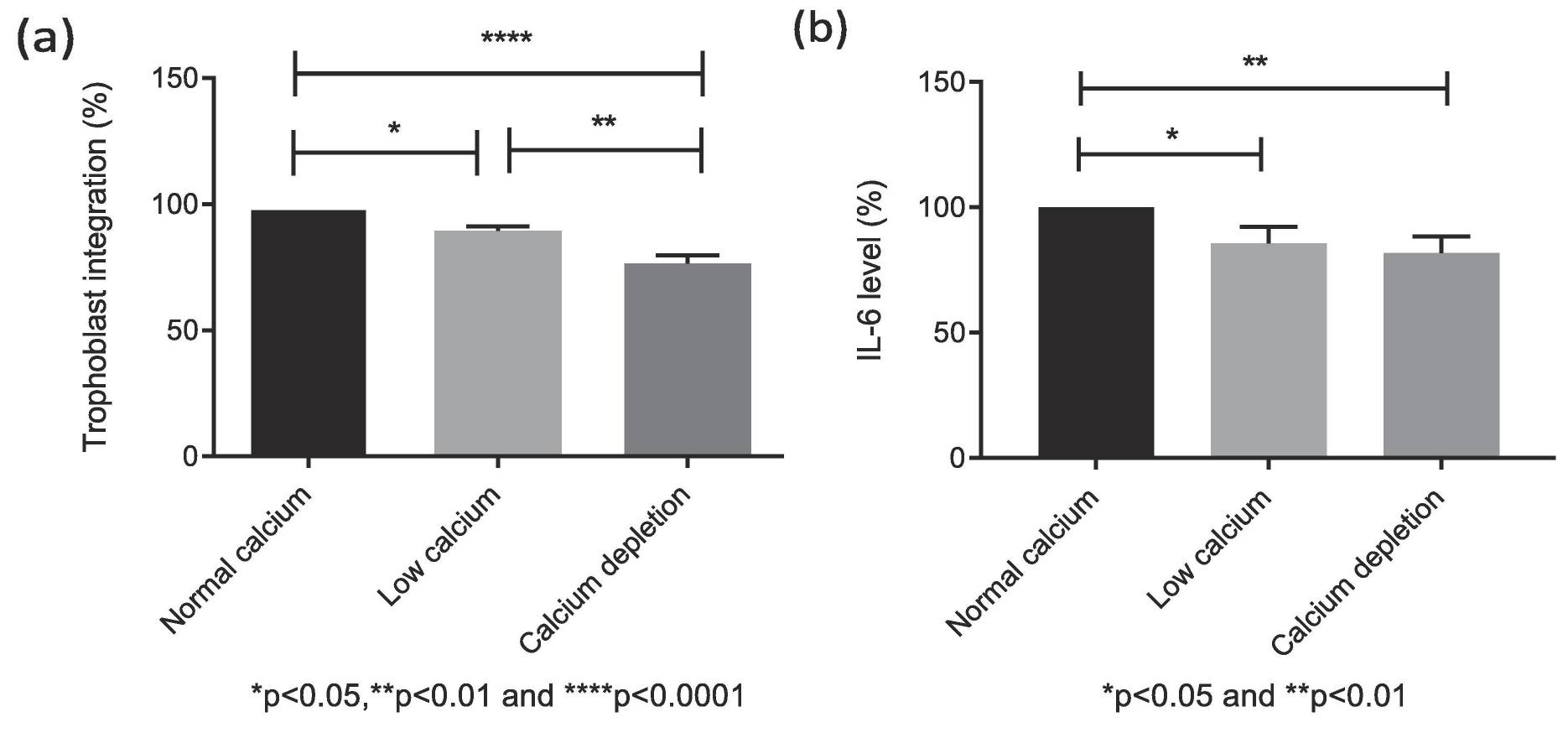 Fig. 1. Effects of calcium concentrations on the IL-6 production in the co-cultured conditioned medium (Xu B, Makris A, et al., 2020).
Fig. 1. Effects of calcium concentrations on the IL-6 production in the co-cultured conditioned medium (Xu B, Makris A, et al., 2020).
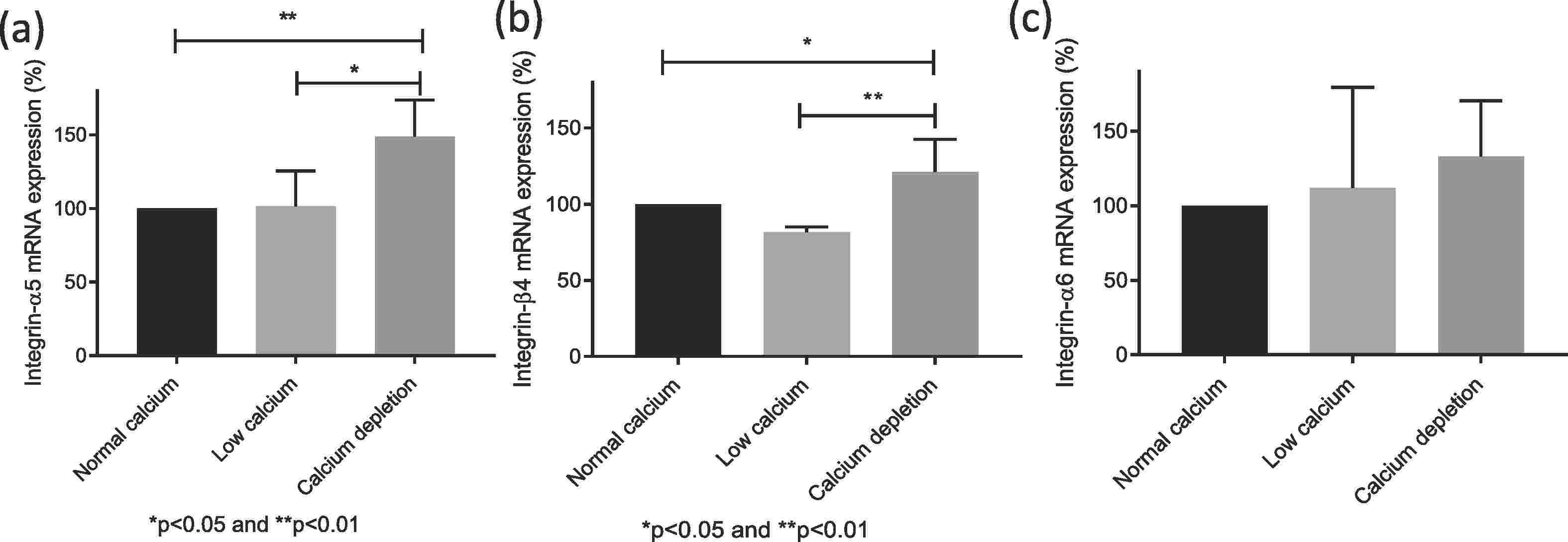 Fig. 2. Relative mRNA expression of integrin α5 (a), β4 (b) and α6 (c) in endothelial/trophoblast co-culture after exposure to different calcium concentrations (Xu B, Makris A, et al., 2020).
Fig. 2. Relative mRNA expression of integrin α5 (a), β4 (b) and α6 (c) in endothelial/trophoblast co-culture after exposure to different calcium concentrations (Xu B, Makris A, et al., 2020).
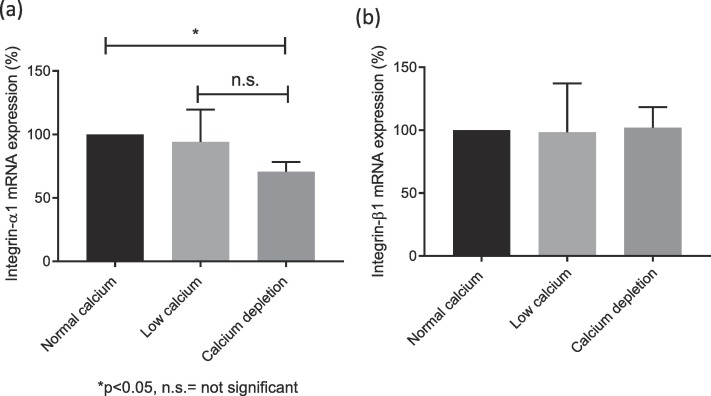 Fig. 3. Relative mRNA expression of integrin α1 (a) and β1 (b) in endothelial/trophoblast co-culture after exposure to different calcium concentrations (Xu B, Makris A, et al., 2020).
Fig. 3. Relative mRNA expression of integrin α1 (a) and β1 (b) in endothelial/trophoblast co-culture after exposure to different calcium concentrations (Xu B, Makris A, et al., 2020).
Angiopoietin-2 Regulated by Progesterone Induces Uterine Vascular Remodeling During Pregnancy
During pregnancy, the uterus undergoes significant neovascularization and remodeling to nourish the embryo. Progesterone influences these processes through angiogenic factors, notably angiopoietin-2 (Ang-2), which plays a role in vascular remodeling.
Park et al. aim to explore the interplay between Ang-2 and uterine vascular remodeling during pregnancy, specifically investigating progesterone's effect on Ang-2 expression. In mouse models, Ang-2 expressed in the uterine endometrium during early pregnancy, mainly in CD31+ blood vessels. In previous studies, uterine vascular remodeling is regulated by progesterone via its receptor, PR. They hypothesized that Ang-2 in the uterus might relate to progesterone. Comparison of PR and Ang-2 expression regions confirmed their co-localization. In vitro experiments with HUtMEC showed that progesterone treatment increases Ang-2 expression, aligning with in vivo data. Using western blotting and ICC, they found PR expression in HUtMEC regardless of progesterone levels (Fig. 4A), with Ang-2 present (Fig. 4B). Blocking PR with RU486 significantly reduced uterine vascular remodeling and Ang-2 levels (Fig. 5A and C). RU486 treatment decreased blood vessels over 300 µm in diameter while increasing those between 100-300 µm (Fig. 5B), indicating PR blockade inhibits vascular remodeling and reduces Ang-2 in endothelial cells.
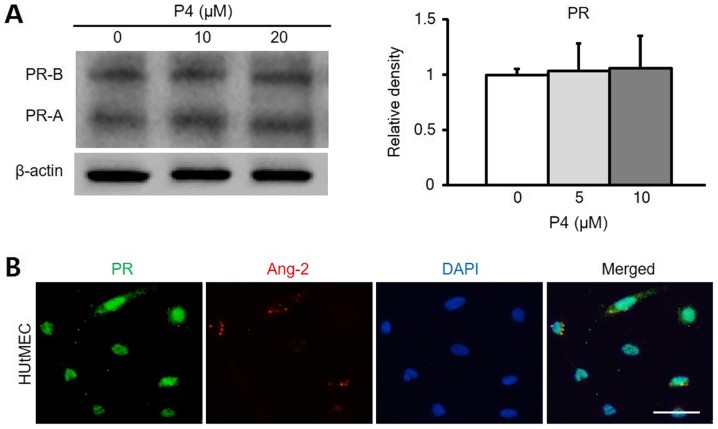 Fig. 4. Ang-2 expression is associated with PR in HUtMECs (Park YG, Choi J, et al., 2020).
Fig. 4. Ang-2 expression is associated with PR in HUtMECs (Park YG, Choi J, et al., 2020).
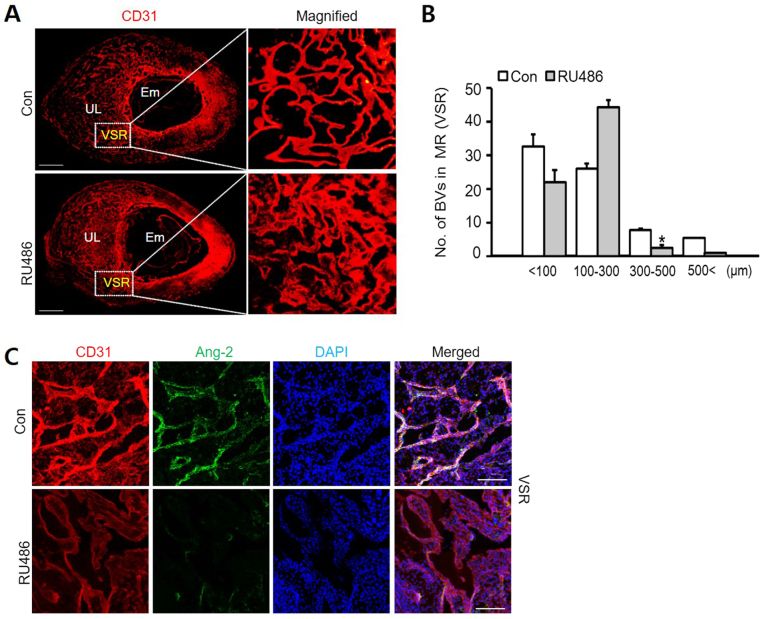 Fig. 5. Vascular remodeling in VSR is decreased by RU486 (Park YG, Choi J, et al., 2020).
Fig. 5. Vascular remodeling in VSR is decreased by RU486 (Park YG, Choi J, et al., 2020).
Ask a Question
Write your own review


