ONLINE INQUIRY

Human Podocyte
Cat.No.: CSC-C0320Z
Species: Human
Source: Kidney
Cell Type: Podocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The human podocyte cell line is a key in vitro model for studying human glomerulopathies. The cell bodies are usually round or oval and have a huge nucleus. Numerous elongated foot processes emerge from the cell wall, twisting and joining to create a branch-like structure. These foot processes are covered by complexes of proteins that adhere to the GBM via adhesion molecules and proteoglycans. In the foot processes, there are many 30-40 nanometre slits and between the slits 4-6 nanometer-thick slit diaphragms define the last obstacle to plasma proteins entering the vascular system.
Podocytes can also secrete extracellular matrix materials, such as collagen and laminin, and aid in extracellular matrix degradation. In accurately balancing synthesis and degradation of the extracellular matrix, podocytes preserve the stability and proper functioning of the glomerular basement membrane. In renal physiology, podocytes receive and broadcast signals, adapt to hemodynamics, and regulate their activity via a cascade of intracellular signaling networks. Podocytes also communicate with other glomerular cells, including endothelial and mesangial cells, to help collectively control the homeostasis of the glomerulus. Since podocyte structural and functional abnormalities can cause proteinuria and hematuria in glomerular disease, detailed studies of the signal transduction and gene expression regulation of Human Podocyte cells are essential to discovering novel treatments for kidney disease.
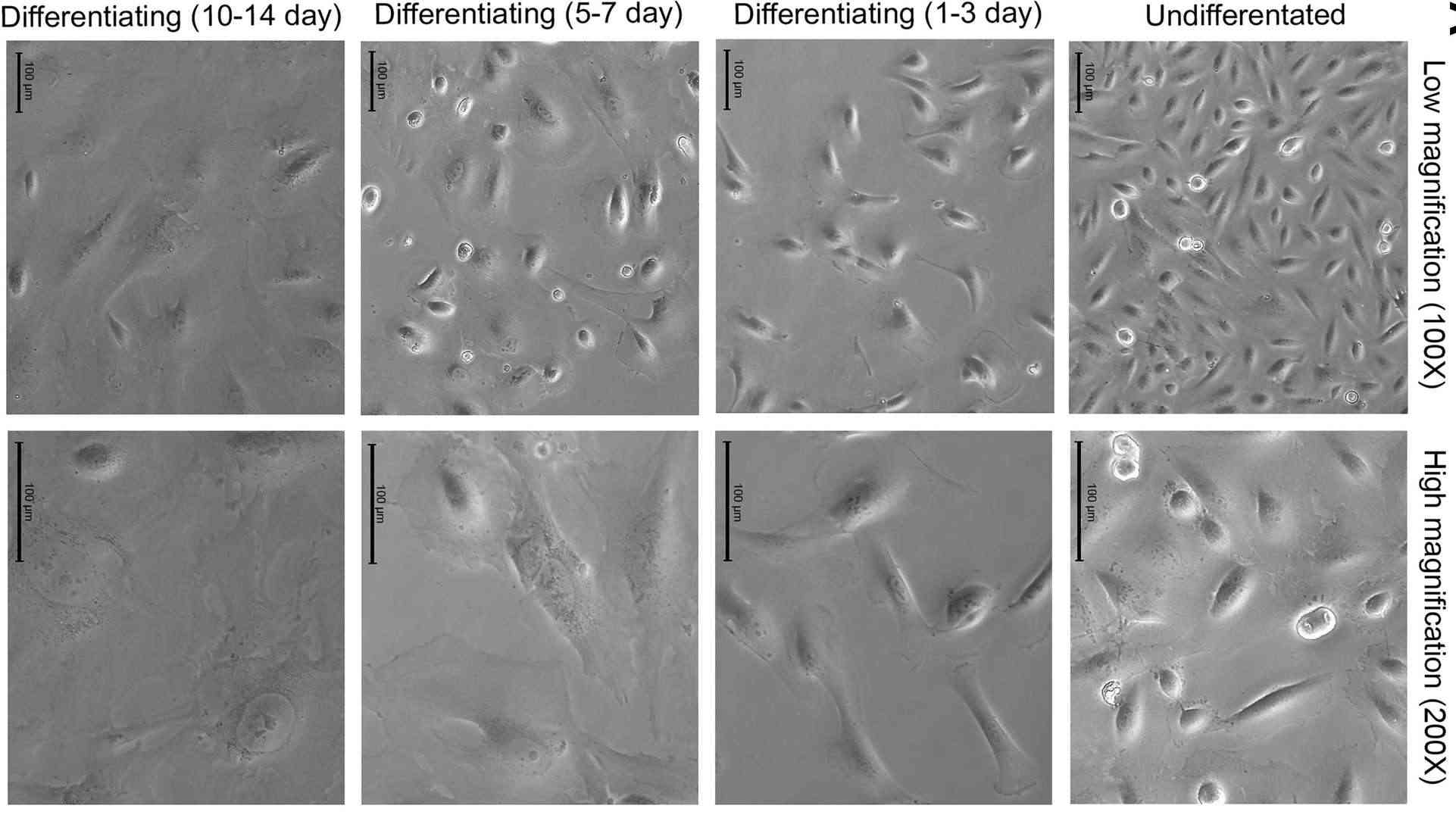 Fig. 1. Identification of differentiated human immortalized podocytes (Martinez-Arroyo O, Ortega A, et al., 2020).
Fig. 1. Identification of differentiated human immortalized podocytes (Martinez-Arroyo O, Ortega A, et al., 2020).
Tacrolimus Prevents TWEAK-Induced PLA2R Expression in Cultured Human Podocytes
Primary membranous nephropathy (MN) is the leading cause of nephrotic syndrome among nondiabetic Caucasian adults and is usually caused by antibodies against the podocyte antigen membrane M-type phospholipase A2 receptor (PLA2R). While calcineurin inhibitors like tacrolimus are standard treatments, they often lead to disease recurrence upon withdrawal. In this regard, whether calcineurin inhibitors modulate podocyte PLA2R expression has not been explored so far. Cuarental's team investigated the factors influencing PLA2R expression in podocytes and the impact of tacrolimus especially focusing on the TNF receptor superfamily member TNFRSF12a (Fn14) and its ligand TWEAK.
The results showed that, in human membranous nephropathy (MN), glomerular expression of TNFRSF12A (encoding Fn14) is significantly upregulated, more so than TNFRSF21. This upregulation was confirmed via immunohistochemistry, showing increased Fn14 levels and NFκB activation in podocytes. Furthermore, systemic administration of TWEAK, the ligand for Fn14, was found to increase PLA2R expression in podocytes, indicating a potential pathway for inflammation-mediated promotion of this nephropathy-related receptor (Fig. 1). Next, they examined TWEAK's effect on PLA2R expression in human podocytes, noting an increase in PLA2R mRNA and protein within 6 hours (Fig. 2A and B). TWEAK also elevated IRF4 and NFKB1 mRNA levels in cultured podocytes (Fig. 2C and D). These genes are associated with MN via GWAS studies. TWEAK similarly boosted NFKB1 in tubular cells but did not affect PLA2R or IRF4 mRNA in these cells. Tacrolimus inhibited TWEAK-induced upregulation of PLA2R, NFKB1, and IRF4 in podocytes, reflecting its role in suppressing MN-related gene expressions (Fig. 2).
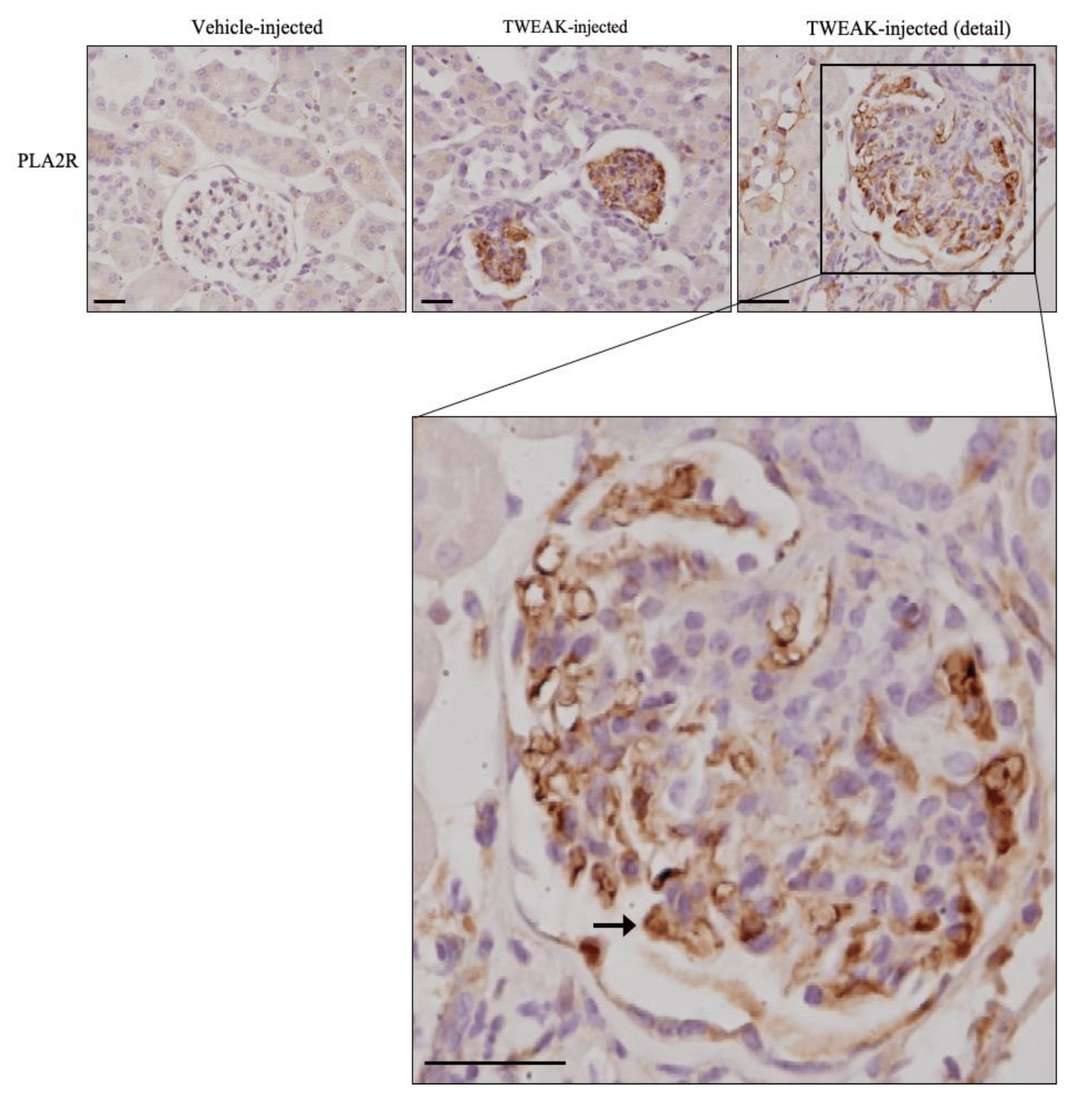 Fig. 1. TWEAK increases PLA2R expression in podocytes in vivo (Cuarental L, Valiño-Rivas L, et al., 2020).
Fig. 1. TWEAK increases PLA2R expression in podocytes in vivo (Cuarental L, Valiño-Rivas L, et al., 2020).
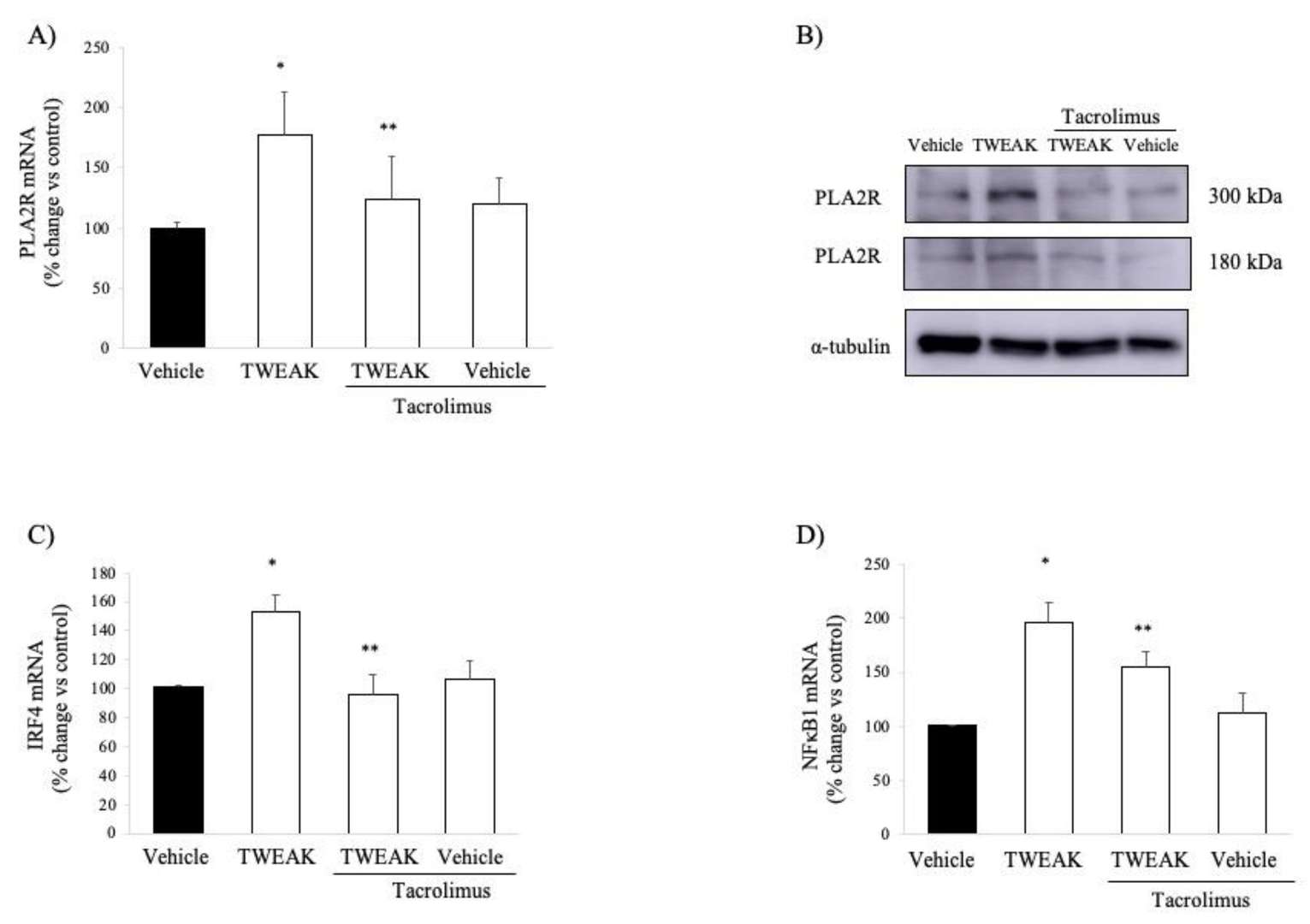 Fig. 2. TWEAK increases PLA2R expression in cultured human podocytes: impact of tacrolimus (Cuarental L, Valiño-Rivas L, et al., 2020).
Fig. 2. TWEAK increases PLA2R expression in cultured human podocytes: impact of tacrolimus (Cuarental L, Valiño-Rivas L, et al., 2020).
Silencing of TCF7 Ameliorated HG-Triggered Injury in Podocytes
Diabetic nephropathy (DN) is a severe complication of diabetes characterized by podocyte destruction. Hyperglycemia, the most important contributor to podocyte loss, can lead DN towards diabetic nephropathy. The pathogenicity of DN involves long non-coding RNAs (lncRNAs) such as LncRNA T-cell factor 7 (TCF7) and semaphorin-3A (SEMA3A) implicated in kidney disease. But it is not clear how TCF7's effects on DN are related to SEMA3A.
Jiang's team explored TCF7's role in high glucose podocyte damage and whether it is mediated via the miR-16-5p/SEMA3A pathway. TCF7 expression significantly increased compared with glucose and mannitol groups (Fig. 3a). In order to better characterise the role of TCF7 in diabetic nephropathy, TCF7 was silenced by siRNA (si-TCF7), which knocked down TCF7 expression in high-glucose or non-hyperglycemic podocytes (Fig. 3b and c). High glucose lowered cell viability (Fig. 3d) and resulted in greater apoptosis (Fig. 3e and f) by enhancing Bax and decreasing Bcl-2 (Fig. 3g and h). It also upregulated ROS (Fig. 3i), MDA (Fig. 3j), IL-1 (Fig. 3m), TNF- (Fig. 3n), IL-6 (Fig. 3o) and decreased SOD (Fig. 3k) and CAT (Fig. 3l). TCF7 silencing blocked these podocyte-affecting high glucose levels (Fig. 3d–o).
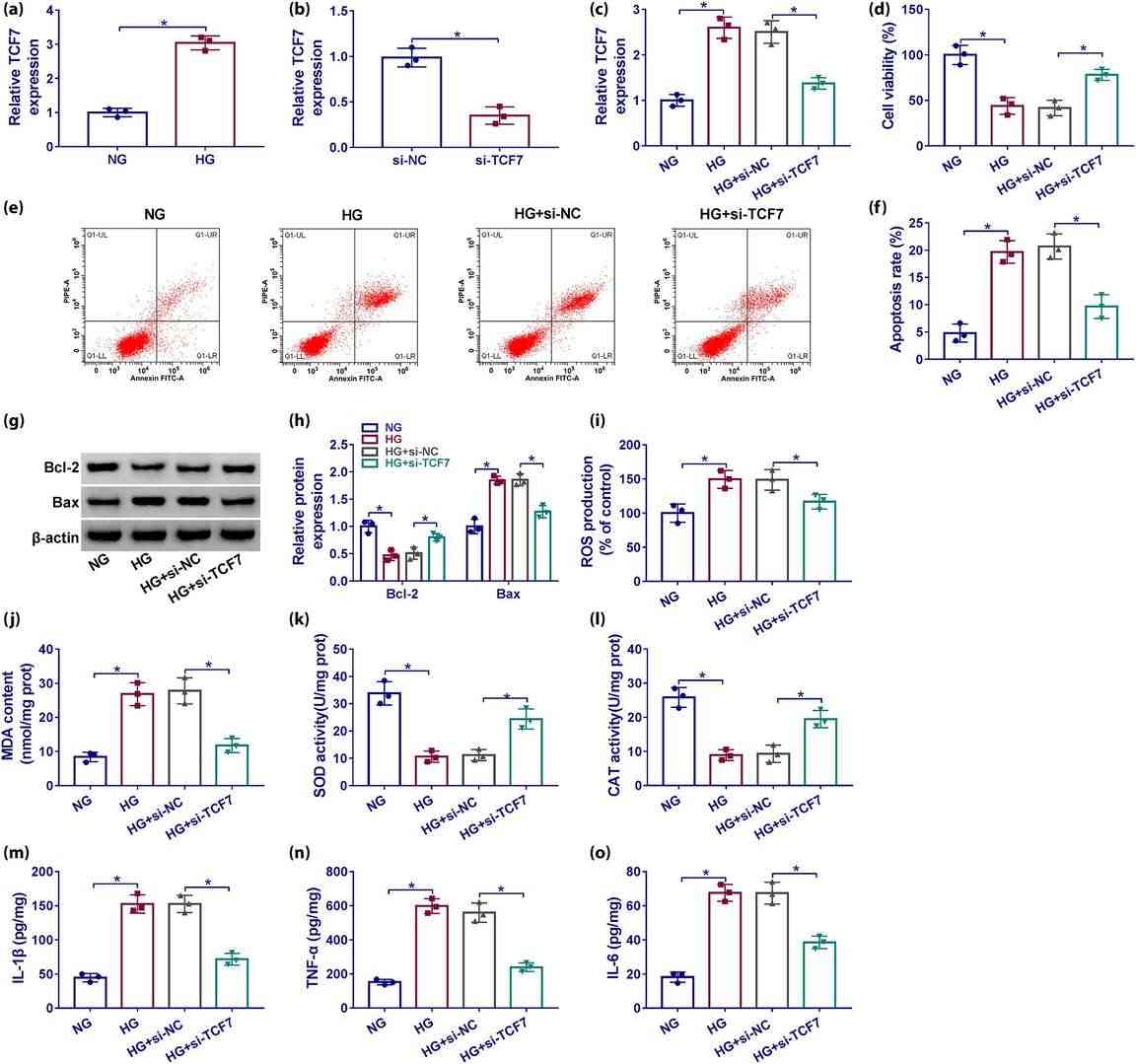 Fig. 3. TCF7 depletion protected podocytes from cytotoxicity induced by high glucose (Jiang Z, Qian L, et al., 2023).
Fig. 3. TCF7 depletion protected podocytes from cytotoxicity induced by high glucose (Jiang Z, Qian L, et al., 2023).
Ask a Question
Write your own review