ONLINE INQUIRY

Human Lung Microvascular Endothelial Cells
Cat.No.: CSC-C4864L
Species: Human
Source: Lung
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Human lung microvascular endothelial cells (HLMVECs) come from healthy human lung tissue, and have flattened polygonal shape with sharp edges. They lay flat on top of one another in a cobblestone pattern, and they have typical epithelial features. When cultured in vitro, HLMVECs can form a thick monolayer and mimic microvascular endothelium well in vivo.
The physiology of lung tissue contains a number of essential functions for HLMVECs. They bind together to create a selectively porous membrane, titling precisely the flow of substances between blood and tissue fluid. It does not only prevent harmful molecules from colonizing lung tissue but also helps oxygen and nutrients to move freely. Also, they have a surface of many coagulation factors and plasminogen activator inhibitors, enabling them to fine-tune blood coagulation and fibrinolysis so that no abnormal event like thrombosis or hemorrhage happens. Moreover, HLMVECs express cell adhesion molecules such as ICAM-1 and VCAM-1, which bind to leukocytes to coordinate adhesion, migration and exocytosis, rendering them indispensable components of pulmonary immune defenses and inflammatory reactions. Because of their special physiological and functional features, HLMVECs are extensively utilized in research areas such as vascular biology, tumorigenesis and metastasis mechanisms, drug screening, and toxicity assessment.
 Fig. 1. Human lung microvascular endothelial cells (Shi Q, Liu H, et al., 2023).
Fig. 1. Human lung microvascular endothelial cells (Shi Q, Liu H, et al., 2023).
Pirfenidone and Nintedanib Inhibit the IL-11-Induced Endothelial to Mesenchymal Transition and Pulmonary Artery Smooth Muscle-Myofibroblast Like Transformations
Idiopathic pulmonary fibrosis (IPF) often leads to pulmonary hypertension (PH), worsening patient prognosis. IL-11 is overexpressed in IPF-PH patients, contributing to pulmonary artery remodeling through endothelial and smooth muscle cell transformations. Current therapies lack effective solutions for vascular remodeling induced by IL-11 in IPF-PH. Nintedanib (NTD) and pirfenidone (PFD) are the only approved drugs for IPF, each with distinct mechanisms of action.
Roger et al. examined the effects of nintedanib and pirfenidone on IL-11-induced pulmonary artery endothelial and smooth muscle cell remodeling. Firstly, they tested their role in inhibiting cellular transitions. In human lung microvascular endothelial cells (HMVECs), rhIL-11 (5 ng/ml) caused an increase in α-SMA and VIM overexpression, transcription of extracellular COL I and growth factors like FGF, PDGF, VEGF, and vasoactive ET-1, while reducing eNOS, VE-cadherin, and FVIII, indicating an EnMT process (Fig. 1A). NTD and PFD can inhibit this process without affecting FGF, NOS3, and FVIII expression. Protein levels mirrored transcription changes; rhIL-11 raised COL I and α-SMA, while decreasing VE-Cadherin. NTD reversed rhIL-11 effects on COL I and VE-Cadherin, and PFD reduced COL I and α-SMA while increasing VE-cadherin (Fig. 1B). In human pulmonary artery smooth muscle cells (HPASMCs), rhIL-11 increased COL I, growth factors, ET-1, and VIM expression. NTD and PFD inhibited the myofibroblast-like transition (Fig. 2A), though NTD did not reduce COL I protein levels (Fig. 2B). PCLS models showed cellular diversity advantages. In healthy PCLS, rhIL-11 increased αSMA, VIM, COL I, CTGF, FN1, and ET-1 expression. NTD and PFD prevented all increases except for CTGF (Fig. 3A). COL I and α-SMA expression and co-localization occurred in both lung parenchyma and arteries. Both drugs decreased COL I expression, localizing α-SMA primarily in arterial muscles, suggesting reduced mesenchymal transition (Fig. 3B).
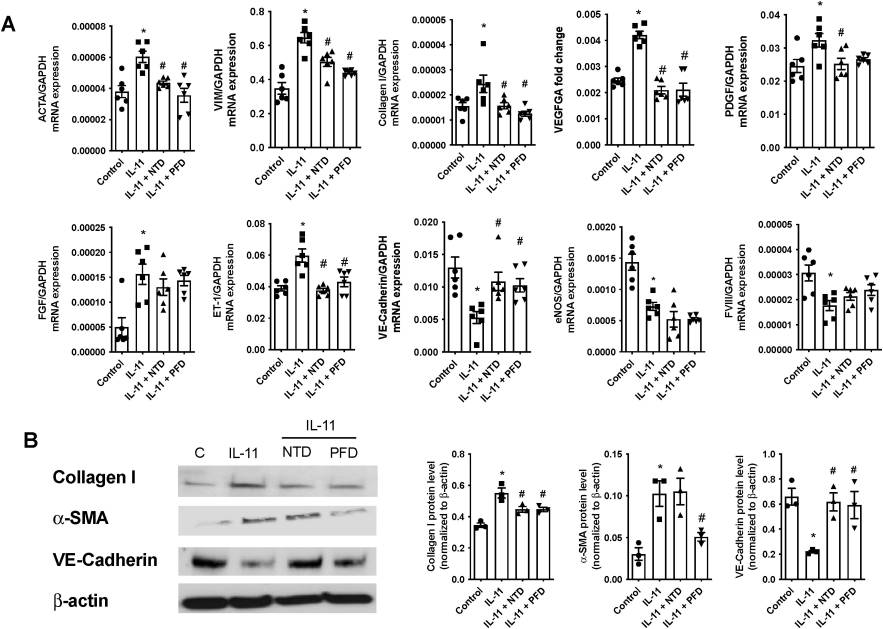 Fig. 1. Nintedanib (NTD) and pirfenidone (PFD) inhibits the IL-11-induce human microvascular endothelial cell (HMVEC) to mesenchymal transition (EnMT) (Roger I, Montero P, et al., 2024).
Fig. 1. Nintedanib (NTD) and pirfenidone (PFD) inhibits the IL-11-induce human microvascular endothelial cell (HMVEC) to mesenchymal transition (EnMT) (Roger I, Montero P, et al., 2024).
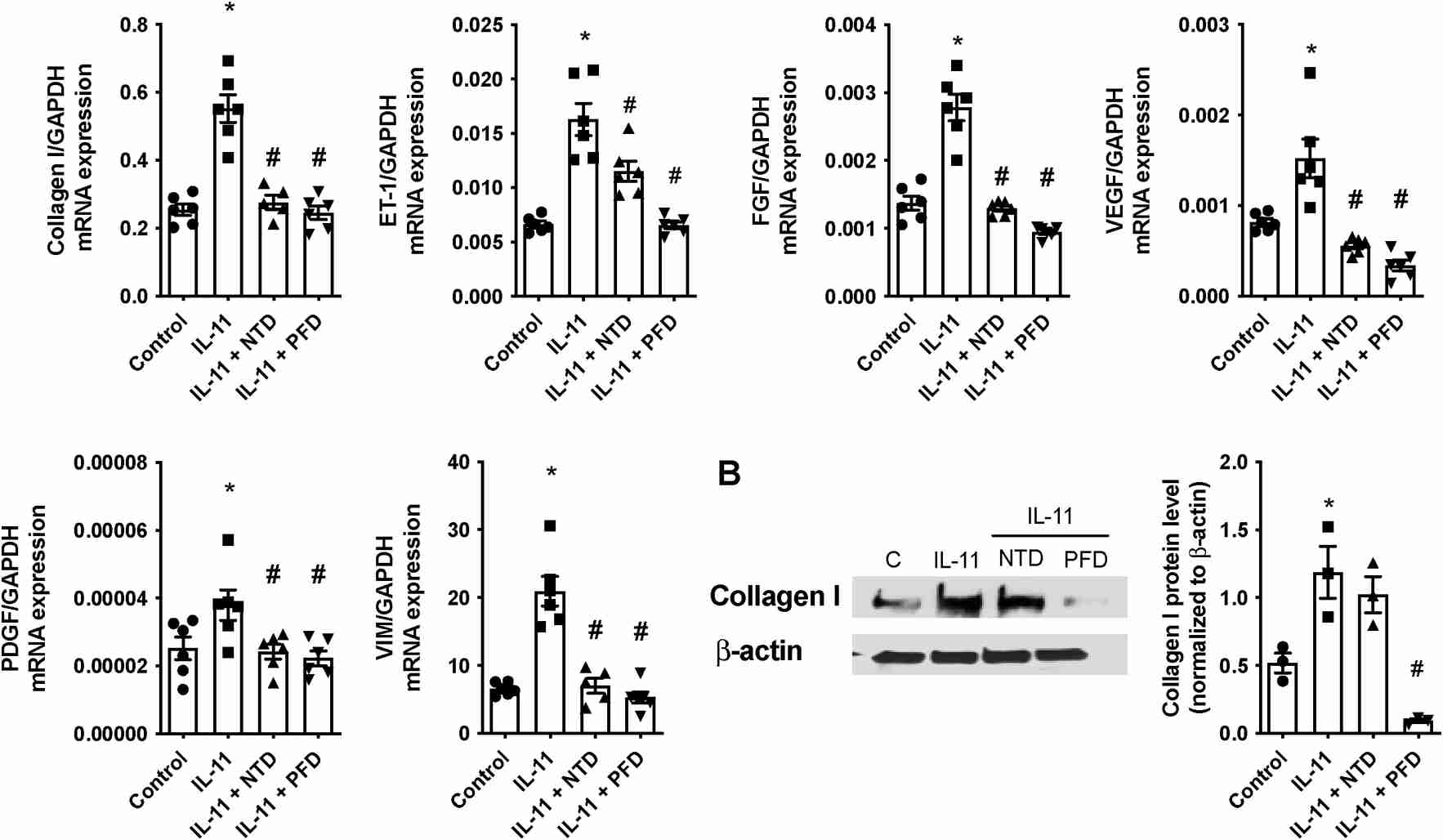 Fig. 2. Nintedanib (NTD) and pirfenidone (PFD) inhibits the IL-11-induce human pulmonary artery smooth muscle cell (HPASMC) to mesenchymal transition (Roger I, Montero P, et al., 2024).
Fig. 2. Nintedanib (NTD) and pirfenidone (PFD) inhibits the IL-11-induce human pulmonary artery smooth muscle cell (HPASMC) to mesenchymal transition (Roger I, Montero P, et al., 2024).
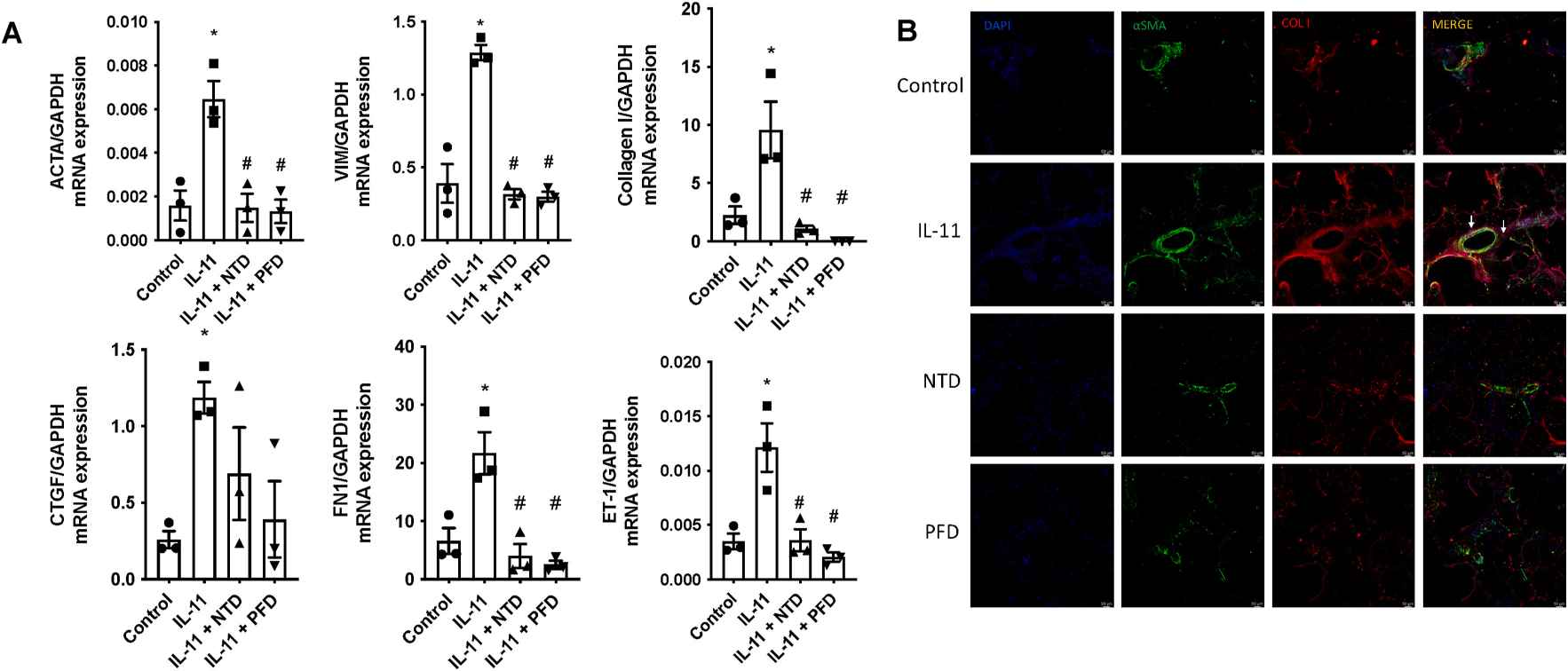 Fig. 3. Nintedanib (NTD) and pirfenidone (PFD) preventing the alteration of vascular remodeling markers induced by IL-11 in precision-cut lung slices (PCLS) (Roger I, Montero P, et al., 2024).
Fig. 3. Nintedanib (NTD) and pirfenidone (PFD) preventing the alteration of vascular remodeling markers induced by IL-11 in precision-cut lung slices (PCLS) (Roger I, Montero P, et al., 2024).
Human Lung Microvascular Endothelial Cells as Potential Alternatives to Human Umbilical Vein Endothelial Cells in Bio-3D-Printed Trachea-Like Structures
Recent advancements in regenerative medicine and tissue engineering have led to the development of new technologies for airway regeneration and the creation of artificial organs. While traditional artificial airway organs rely on scaffolds to maintain structural integrity, these scaffolds carry risks such as infection and reduced biocompatibility. To address these issues, the use of scaffold-free structures via bio-3D printing with cell aggregates, known as spheroids, has been explored. Human umbilical vein endothelial cells (HUVECs) have been instrumental in these structures, but they cannot be sourced from adults.
Taniguchi's team compared three types of cell structures made from chondrocytes and mesenchymal stem cells with HUVECs, human lung microvascular endothelial cells (HMVEC-Ls), and induced pluripotent stem cell (iPSC)-derived endothelial cells to determine their effectiveness as alternatives. These cells were cultured in specific growth media and used to form spheroids. These spheroids consist of a mix of AC, MSCs, and endothelial cells in a predetermined ratio and were created by plating the cell mixture into ultra-low attachment round-bottom plates. After 48 hours, the cells aggregated into spheroids, serving as the building blocks for the 3D printing process. Using the bio-3D printer, these spheroids were automatically skewered into a needle-array according to a pre-designed 3D structure. The needle-array had specific dimensions to ensure precise placement of the spheroids. A total of 384 spheroids were incorporated into a scaffold-free tubular structure. This printed structure was then matured in a bioreactor with perfused medium to ensure proper development and stability (Fig. 4). They found no significant difference in tensile strength was observed between the three groups. Histologically, some small capillary-like tube formations comprising CD31-positive cells were observed in all groups. The number and diameters of such formations were significantly lower in the iPSC-derived endothelial cell group than in other groups. Glycosaminoglycan content was significantly lower in the iPSC-derived endothelial cell group than in the HUVEC group, while no significant difference was observed between the HUVEC and HMVEC-L groups. In conclusion, HMVEC-Ls can replace HUVECs as a cell source for scaffold-free trachea-like structures. However, some limitations were associated with iPSC-derived endothelial cells.
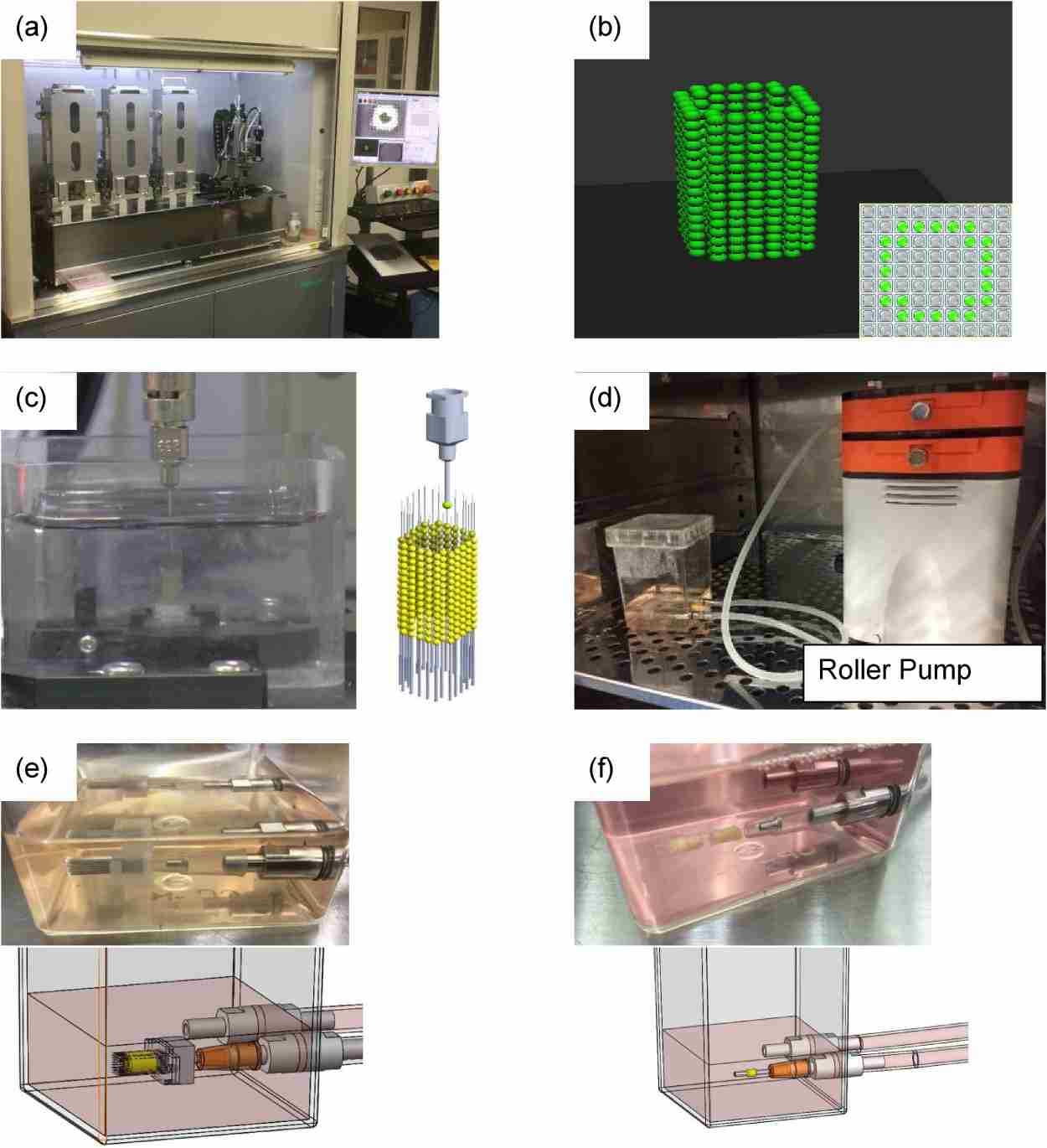 Fig. 4. Bio-3D printing and maturation of 3D-printed structure (Taniguchi D, Matsumoto K, et al., 2020).
Fig. 4. Bio-3D printing and maturation of 3D-printed structure (Taniguchi D, Matsumoto K, et al., 2020).
Umbilical vein endothelial cells are recommended to be coated with 0.1% gelatin, the morphology of the coated cells will be better than the non-coated ones.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Trustworthy
We have been using Creative Bioarray's cellular products and look forward to continuing to develop new ones.
22 Mar 2021
Ease of use
After sales services
Value for money
Write your own review

