ONLINE INQUIRY
Bovine Skeletal Muscle Cells
Cat.No.: CSC-C0524Z
Species: Bovine
Source: Skeletal Muscle
Morphology: Polygonal
Culture Properties: Adherent
Cell Type: Skeletal Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20°C.
Bovine skeletal muscle cells are multinucleated cells formed by the fusion of myoblasts, and they are among the larger cell types in the body. These elongated, fiber-like cells have the ability to contract when stimulated by internal or external signals and display alternating light and dark bands as a structural characteristic, primarily composed of myofibrils. Their core functions include supporting essential life activities such as respiration, movement, and temperature regulation, while also facilitating muscle growth, repair, and regeneration. Due to high energy demands, these cells contain numerous mitochondria to produce sufficient ATP.
At different growth stages, bovine skeletal muscle cells exhibit distinct metabolic characteristics. Myofibril formation drives embryonic muscle fiber development but adult muscle fibers increase their diameter to meet demand. During the differentiation process of bovine skeletal muscle cells multiple signaling pathways such as insulin-like growth factor (IGF), myostatin and the Wnt/β-catenin pathway perform regulatory functions. These cells perform essential functions in muscle regeneration and pathology. Multiple factors impact the metabolic processes and differentiation behavior of bovine skeletal muscle cells in muscular dystrophy and degenerative diseases according to research studies. In the field of bioengineering, bovine skeletal muscle scaffolds are used to simulate the growth and repair of muscle tissue. In genetic research, gene editing technologies are employed to regulate their growth and differentiation to enhance meat quality and disease resistance.
Vitamin K2 Improves Proliferation of Bovine Skeletal Muscle Cells In Vitro
Muscle function relies on regeneration capabilities, which decline with age and disease, reducing functional capacity. Vitamin K2's role in muscle regulation and function remains unclear, despite documented presence in bovine muscles. Pedersen's team used primary bovine muscle cells cultured in vitro to evaluate vitamin K2's (MK-4) effects on migration, proliferation, and differentiation.
The experiments showed no change in ATP production with MK-4 addition, indicating cell viability and metabolic activity (Fig 1A). MK-4 is known to enhance mitochondrial electron transfer, improving ATP production. This implies vitamin K2 may boost mitochondrial function, addressing mitochondrial issues. Skeletal muscles hold the body's highest mitochondrial content, and their decline is linked to muscle diseases and aging. Mitochondrial imbalance is a key factor in age-related muscle mass and strength loss (sarcopenia). Lactate dehydrogenase (LDH), released during tissue damage, serves as a marker for muscle pathology. MK-4 reduced LDH release slightly (Fig 1B), indicating benefits to muscle cells. Thus, MK-4 may positively affect muscle function. After 72 hours of MK-4 treatment, muscle cell proliferation increased significantly compared to controls, with 10 μM MK-4 being most effective (Fig. 1B and 2A). This concentration was used in subsequent experiments. MyoD is crucial for cell proliferation and early differentiation, while myogenin supports differentiation in myogenesis. Proteins desmin and MYH8 are marker proteins of differentiated cells. We measured the expression of myoD, myogenin, myh8, and desmin in cells treated with 10 μM MK-4 for 3 days (Fig 2B). Results showed increased myoD and myogenin expression, with myoD significantly upregulated. Unlike osteoblast cells, where vitamin K2 boosts differentiation, MK-4 slows myotube formation and reduces gene expression of muscle markers (Fig. 2C and 2D). Further research is needed to explore vitamin D and K interactions.
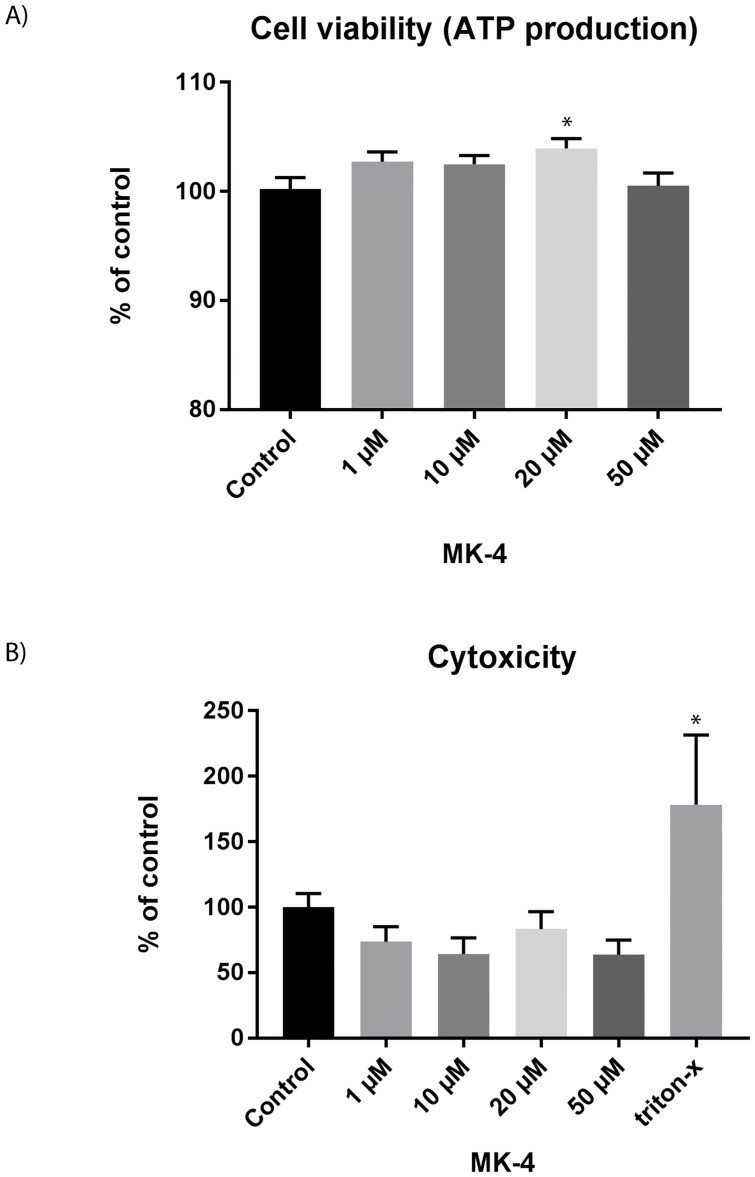 Fig. 1. Viability and cytotoxicity upon MK-4 incubation for 72 h (Rønning S B, Pedersen M E, et al., 2018).
Fig. 1. Viability and cytotoxicity upon MK-4 incubation for 72 h (Rønning S B, Pedersen M E, et al., 2018).
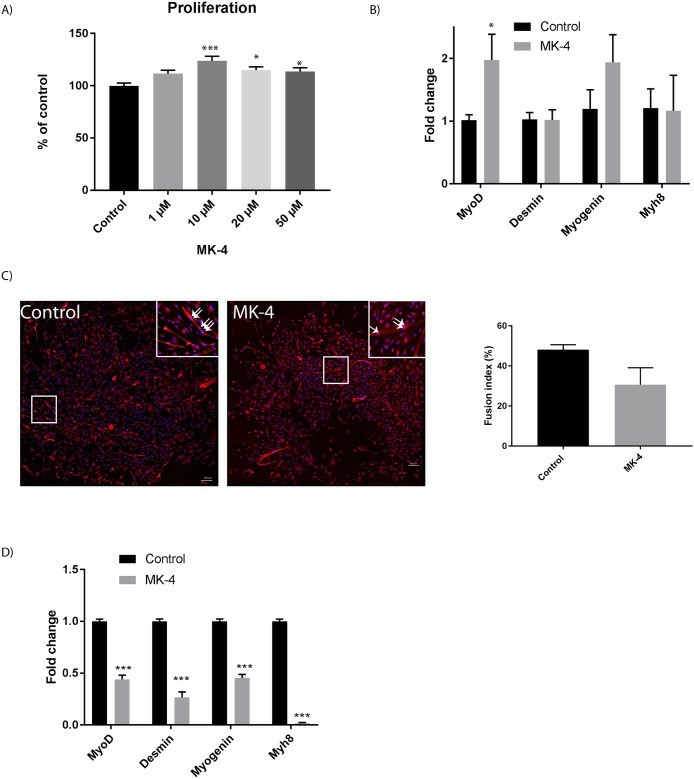 Fig. 2. Effect of MK-4 incubation on muscle proliferation and differentiation (Rønning S B, Pedersen M E, et al., 2018).
Fig. 2. Effect of MK-4 incubation on muscle proliferation and differentiation (Rønning S B, Pedersen M E, et al., 2018).
Inactivation of the MSTN Alleviated Senescence Caused by the Long-Term Culture of bSMCs
The myostatin (MSTN) gene, known for regulating skeletal muscle growth, is linked to muscle wasting due to aging. While the MSTN gene's connection to age-related decline is studied in rodents, its impact on muscle loss in other animals remains unclear.
To assess MSTN inactivation's impact on long-term cultured bovine skeletal muscle cells (bSMCs), Yang's team used WT and MSTN knockout (MT-KO) bSMCs. MT-KO cells showed significantly reduced MSTN expression compared to WT (Fig. 3a). They established aging models for both, noting that after many passages, WT and MT-KO cells became larger, flat, and less plastic (Fig. 3b and c). WT cells were passaged up to 30 generations, while MT-KO cells lasted nearly 40 generations (Fig. 3d). Advances in understanding senescent cells led researchers to propose the multi-marker approach for evaluating senescence, assessing SA-β-gal activity, cell proliferation markers, and p16, p21 expression. They found telomere length decreased with passage number, with WT cells having shorter telomeres than MT-KO cells (Fig. 4a). SA-β-gal positivity increased in WT compared to MT-KO, with decreased ki67 positivity and a reduced G1/S cell cycle phase, alongside increased p16 expression (Fig. 4b-e). These results indicate MSTN downregulation delays bovine muscle cell senescence.
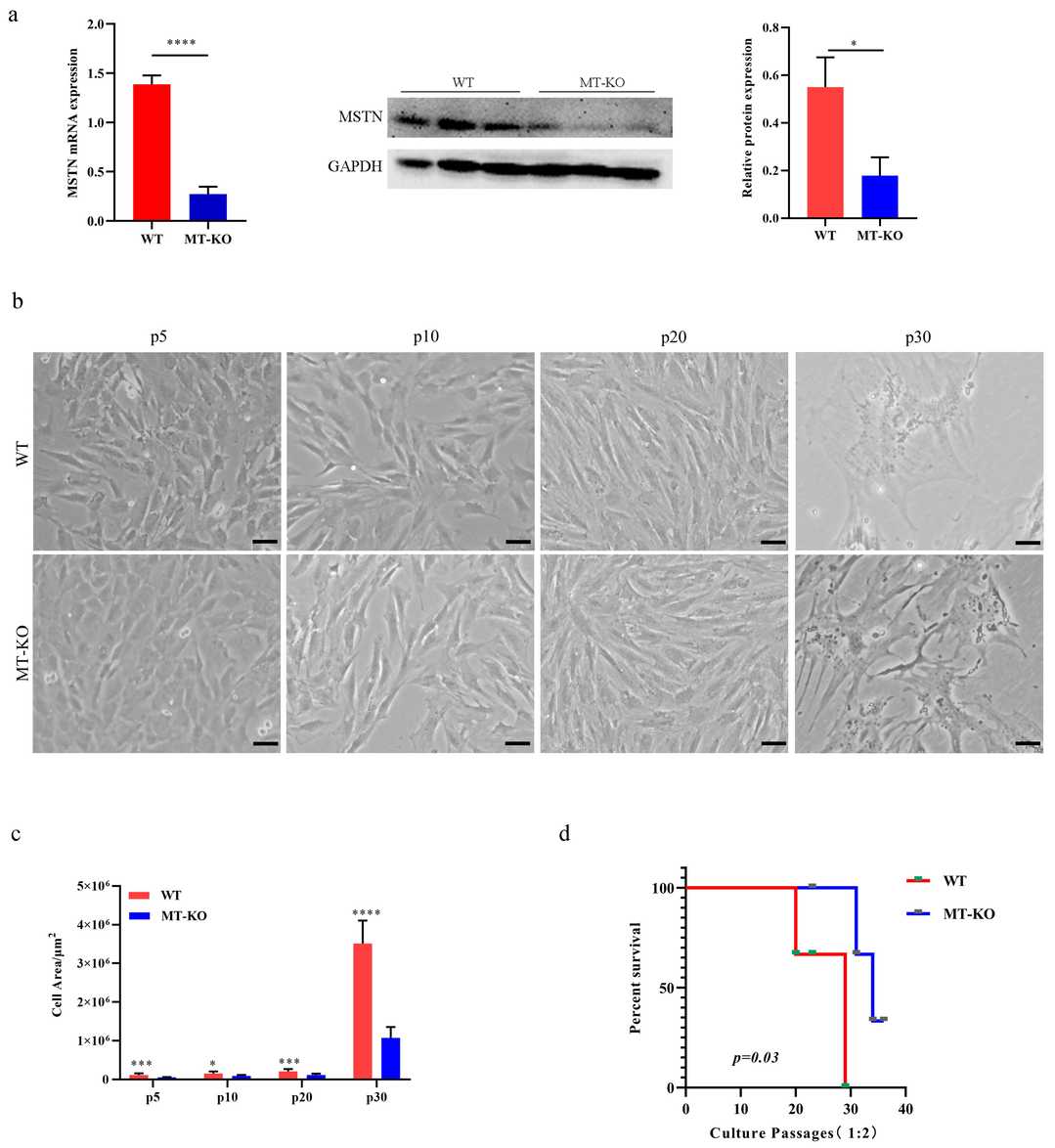 Fig. 3. Characteristics of long-term cultured inactivation of the MSTN bSMCs (Yang M, Gao L, et al., 2024).
Fig. 3. Characteristics of long-term cultured inactivation of the MSTN bSMCs (Yang M, Gao L, et al., 2024).
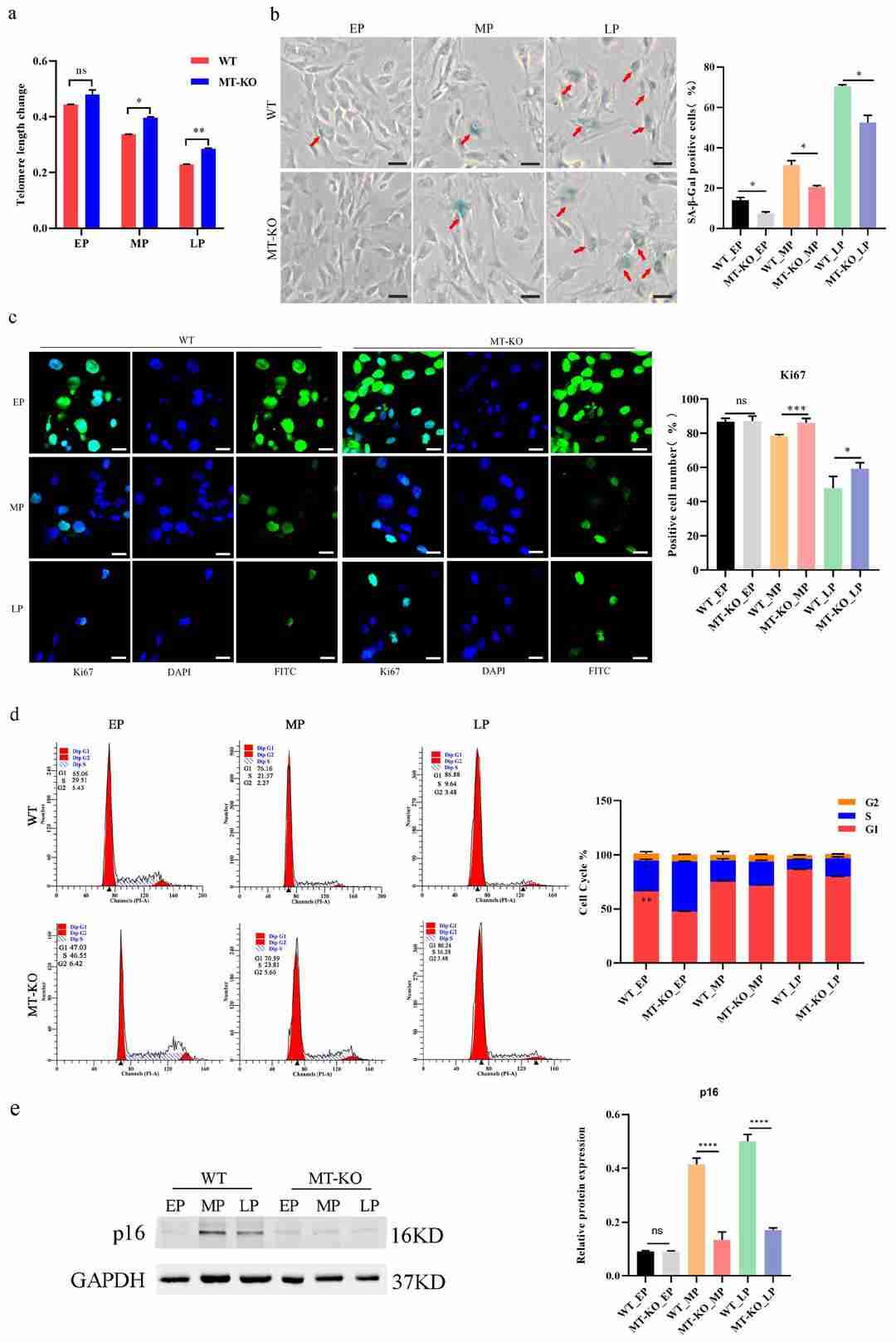 Fig. 4. Hallmarks of the senescence phenotype in long-term-cultured bSMCs (Yang M, Gao L, et al., 2024).
Fig. 4. Hallmarks of the senescence phenotype in long-term-cultured bSMCs (Yang M, Gao L, et al., 2024).
Ask a Question
Write your own review