ONLINE INQUIRY
Bovine Corneal Endothelial Cells
Cat.No.: CSC-C4754Z
Species: Bovine
Source: Cornea; Eye
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C
Bovine Corneal Endothelial Cells (BCECs) originate from the endothelial cell layer of the bovine cornea, the single-cell layer that runs along the inside surface of the bovine cornea and enters the anterior chamber's aqueous humor, separating the corneal stroma from the aqueous humor. By acting as metabolic pumps and barrier proteins, they stop water from reaching the corneal tissue and keep the cornea clear. The BCECs also act in blocking and removing active thrombin, suggesting their role in the anticoagulation process. Moreover, they carry glucose and amino acids from the aqueous humor into the stroma, thereby providing the substances required for metabolism of corneal cells.
BCECs in vitro possess good proliferation and can develop morphologies similar to corneal endothelial cells in the body. Researchers have found that growth factors, like FGF and EGF, significantly enhance BCEC proliferation. When BCECs confluence, their shape and phenotype mimic those of corneal endothelial cells in living tissue. In addition, BCECs are marked by cell markers such as corneal endothelial specific ion channel proteins (AQP1 - Aquaporin 1) and intercellular junction proteins (ZO-1 - Zonula Occludens-1), which are useful for cell detection and purity monitoring.
BCECs are commonly used in drug-screening and toxicology research to cure corneal pathologies and studies of corneal endothelial diseases (Fuchs' endothelial corneal dystrophy, for instance). In addition, BCECs are ideal models for ion transport, intercellular junctional dynamics, and oxidative stress response.
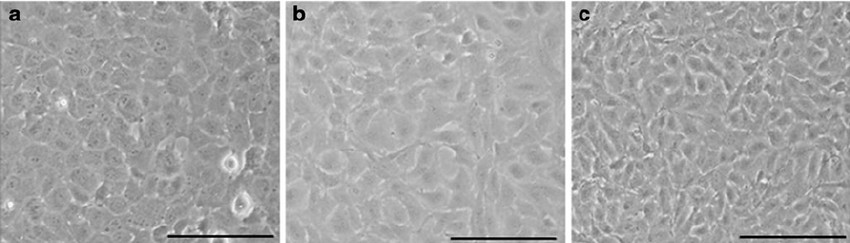 Fig. 1. Primary (a), P10 (b) and P40 (c) bovine corneal endothelial cells showed typical corneal endothelial morphology (Gao Y, Zhou Q, et al., 2010).
Fig. 1. Primary (a), P10 (b) and P40 (c) bovine corneal endothelial cells showed typical corneal endothelial morphology (Gao Y, Zhou Q, et al., 2010).
Dendrimer-coated SPIONs Showed no Toxicity and Enhanced BCEC Migration
Magnetic nanoparticles (MNPs), particularly superparamagnetic iron oxide nanoparticles (SPIONs), are widely used in biomedical applications such as diagnostics and therapeutics. Recently, magnetic nanoparticle applications in ocular systems, such as corneal endothelial cell (CEC) migration and proliferation under external magnetic forces, have received scholarly attention. However, achieving uniform cell distribution on corneal surfaces remains a challenge. Hatamie's team aimed to address this issue by synthesizing dendrimer-functionalized SPIONs and using an electromagnet device to enhance bovine corneal endothelial cell (BCEC) migration, energy metabolism, and symmetric distribution.
The MTT cytotoxicity assay evaluated the effects of five concentrations of D-SPIONs on BCECs over 24, 48, and 72 hours (Fig. 1). No significant toxicity was observed for concentrations up to 100 μg/mL at 24 hours, with cell viabilities of 68% and 65% at 48 and 72 hours, respectively. While iron oxide nanoparticles are generally biocompatible, they showed slight cytotoxicity at higher concentrations. Cell migration, essential for wound healing, was suppressed in BCECs treated with D-SPIONs compared to control cells. BCECs treated with 1 μg/mL D-SPIONs exhibited faster migration than those treated with 10 μg/mL (Fig. 2). Increased nanoparticle agglomeration and cell uptake at higher concentrations likely slowed migration. Under a magnetic field in tapping mode, wound closure with 1 μg/mL D-SPIONs took just 10 hours, faster than in controls. Magnetic vortex patterns were observed in BCECs treated with both 1 and 10 μg/mL D-SPIONs under the magnetic field (Fig. 3b)
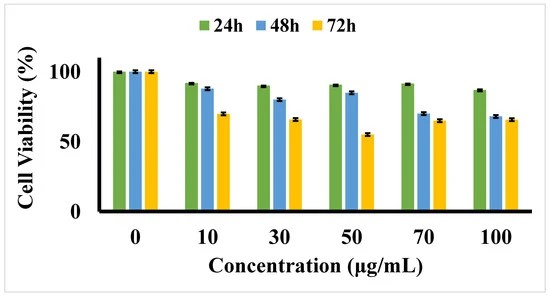 Fig. 1. Cell viability MTT assay for varying concentrations of D-SPIONs on BCECs over 24, 48, and 72 h (Hatamie S, Shih P, et al., 2021).
Fig. 1. Cell viability MTT assay for varying concentrations of D-SPIONs on BCECs over 24, 48, and 72 h (Hatamie S, Shih P, et al., 2021).
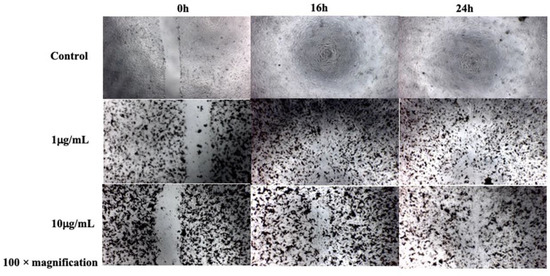 Fig. 2. Migration assay of BCECs treated under varying concentrations of D-SPIONs over 16 and 24 h (Hatamie S, Shih P, et al., 2021).
Fig. 2. Migration assay of BCECs treated under varying concentrations of D-SPIONs over 16 and 24 h (Hatamie S, Shih P, et al., 2021).
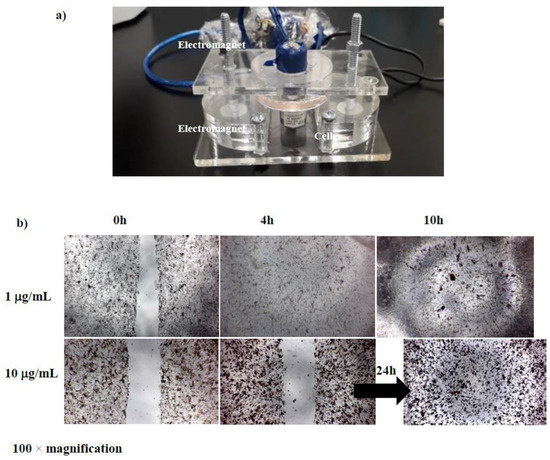 Fig. 3. cell migration assay of BCECs containing different concentrations of D-SPIONs under a gradient magnetic field (Hatamie S, Shih P, et al., 2021).
Fig. 3. cell migration assay of BCECs containing different concentrations of D-SPIONs under a gradient magnetic field (Hatamie S, Shih P, et al., 2021).
Detachment of Bovine Corneal Endothelial Cell Sheets by Cooling-Induced Surface Hydration of Poly[(R)-3-Hydroxybutyrate]-Based Thermoresponsive Copolymer Coating
Cell sheet technology (CST) is an innovative scaffoldless tissue engineering method that preserves cell-to-cell junctions and ECM components, unlike conventional enzymatic harvesting. However, existing CST methods often use complex covalent grafting. Soh's team developed a non-covalent surface coating using triblock copolymers with a hydrophobic PHB core and poly(N-isopropylacrylamide) (PNIPAAm) ends to simplify the culture and detachment of bovine corneal endothelial cell sheets.
They tested the cell sheet detachment capability of the coated substrates. BCECs were seeded at 90,000 cells per well. After three days, they were cooled with 1X PBS at 4°C for 20 minutes. As shown in Figure 4, there were no changes in the cell monolayer for the uncoated control and PNIPAAm-coated substrates. However, on the PNIPAAm-PHB-PNIPAAm (NHN) (100-23-100)-coated substrate, the cell monolayer detached as an intact sheet, indicated by a red arrow. This indicates that the triblock copolymer's phase transition at 0.0157 mg cm−2 coating density changed the surface energy enough to detach the cell sheet. PNIPAAm failed to detach cells likely due to a lack of a hydrophobic anchor. Cooling can lower metabolic activity and protein synthesis, potentially affecting viability. However, viability tests showed no significant difference between cooled and non-cooled cell sheets (Fig. 5). This provides promising evidence for future in vivo transplantation studies.
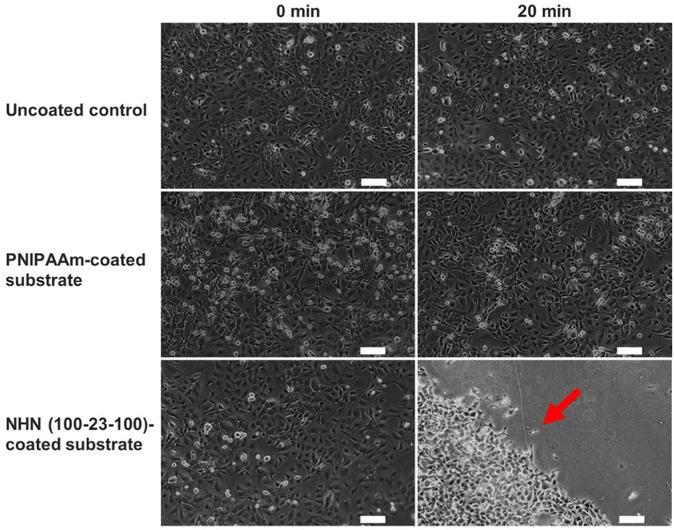 Fig. 4. Phase contrast images of cultured bovine endothelial cells at 0 min and 20 min after cooling at 4°C for uncoated substrate, PNIPAAm-coated substrate, and NHN(100-23-100)-coated substrate (Soh WWW, Zhu J, et al., 2022).
Fig. 4. Phase contrast images of cultured bovine endothelial cells at 0 min and 20 min after cooling at 4°C for uncoated substrate, PNIPAAm-coated substrate, and NHN(100-23-100)-coated substrate (Soh WWW, Zhu J, et al., 2022).
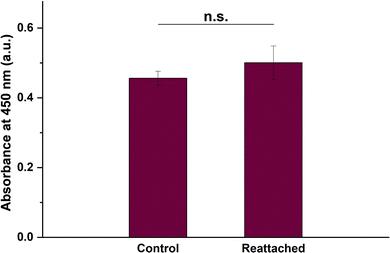 Fig. 5. Cell viability of the BCE cell sheet after detachment by cooling treatment and reattachment as quantified using the CCK-8 assay (Soh WWW, Zhu J, et al., 2022).
Fig. 5. Cell viability of the BCE cell sheet after detachment by cooling treatment and reattachment as quantified using the CCK-8 assay (Soh WWW, Zhu J, et al., 2022).
Ask a Question
Write your own review