ONLINE INQUIRY

Rat Brain Hippocampus Astrocytes
Cat.No.: CSC-C8055L
Species: Rat
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The hippocampus is a brain region essential to learning, memory and spatial navigation. The rat brain hippocampus astrocyte cell line is one of the largest types of glial cells and comes from the rat hippocampi. They have the characteristic stellate shape, and they're multipolar, with lots of longer and shorter projections from the cell body. Such projections mesh together to create complicated network structures. Their thin, well-branched projections allow for comprehensive material transfer, signaling and connectivity with other cells in the environment.
Widely distributed in the interstitial spaces between neurons, these astrocytes are almost entirely matched with all other neuron types in the hippocampus: pyramidal cells and granule cells. They surround neuronal cell bodies, dendrites and synapses, forming a microenvironment that supports and regulates neuronal activity. Astrocytes are involved in almost every function of the central nervous system (CNS): from synapse assembly and development, to the homeostasis of ions and neurotransmitters, to blood-brain barrier regulation, to neuronal metabolism. In clinical studies, rat brain hippocampus astrocytes are often used to investigate the workings of CNS health and disease. They provide key models for the study of neural development, regeneration and neurodegenerative diseases including Alzheimer's and Parkinson's. This cell line also supports research into neuroinflammation, repair of brain injury, and drug metabolism and neurotoxicity in the brain.
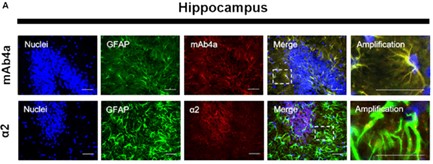 Fig. 1. Rat brain hippocampus astrocytes. Nuclei were stained with Hoechst, GFAP stained astrocytes are green and mAb4a/α2 immunoreactivity is red (Morais TP, Coelho D, et al., 2021).
Fig. 1. Rat brain hippocampus astrocytes. Nuclei were stained with Hoechst, GFAP stained astrocytes are green and mAb4a/α2 immunoreactivity is red (Morais TP, Coelho D, et al., 2021).
Infrasound Downregulated the Expression of FGFR1 in Astrocytes
Infrasound is a public health issue, as it causes vibroacoustic disease (VAD) in systems such as the central nervous system (CNS). Earlier research correlated infrasound-induced CNS damage with the activation of astrocytes and neuroinflammation. Shi et al. exposed Sprague-Dawley rats and cultured astrocytes to 16 Hz, 150 dB infrasound. And examined the effect of FGF2 on astrocyte activation and inflammation through Western blot, immunofluorescence and liquid chip techniques.
In order to establish FGF2/FGFR1's role in infrasound-induced inflammation in astrocytes (rat hippocampus astrocytes), they detected FGFR1 changes after exposure. FGFR1 and GFAP were co-localized, and granular FGFR1 occupied GFAP+ astrocyte membranes (Fig. 1). After exposure to infrasound, astrocytic activation was confirmed by increased GFAP expression (Fig. 2A and C). Expression of FGFR1 significantly decreased 3 and 7 days after exposure compared with controls (Fig. 2A and B). FGFR1+/GFAP+ cells also declined 7 days after exposure (12.00 ± 4.00) when compared with controls (73.00 ± 7.00) (Fig. 2A and D). Primary cultured astrocytes displayed decreased expression of FGFR1 and FGFR1+/GFAP+ cells 12 h (2.75 ± 0.48) and 24 h (1.81 ± 0.38) after exposure, compared with the control (4.61 ± 0.79) (Fig. 2E and F). These findings show in vivo and in vitro declines in astrocyte-expressed FGFR1 following infrasound.
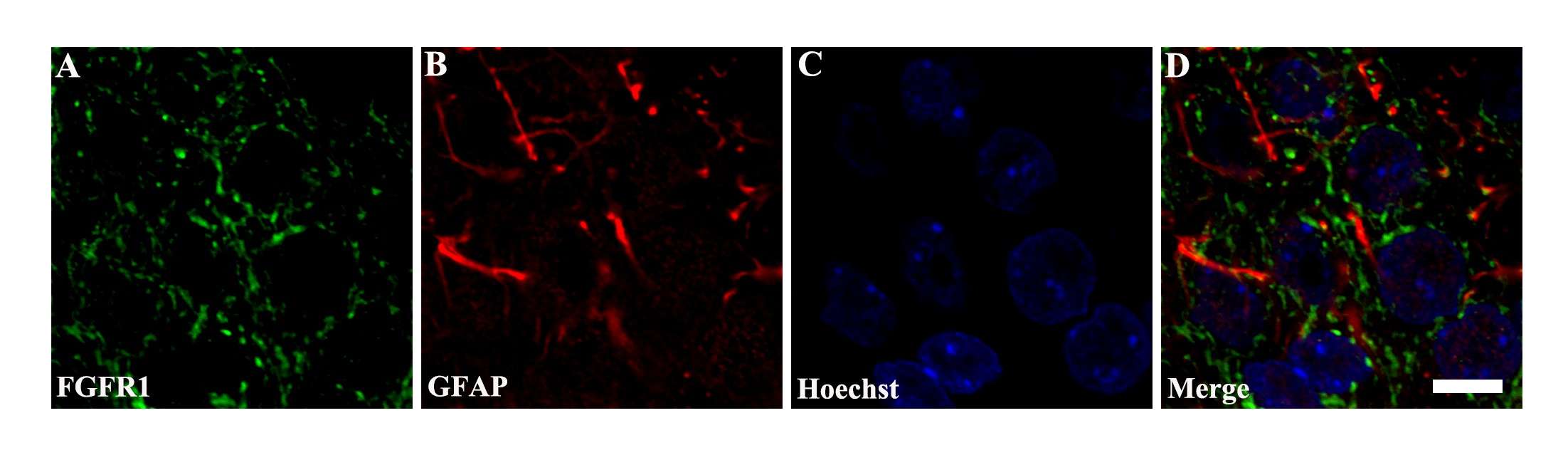 Fig. 1. Co-localization of FGFR1 and GFAP around the CA1 region of hippocampus (Shi J, Shi M, et al., 2018).
Fig. 1. Co-localization of FGFR1 and GFAP around the CA1 region of hippocampus (Shi J, Shi M, et al., 2018).
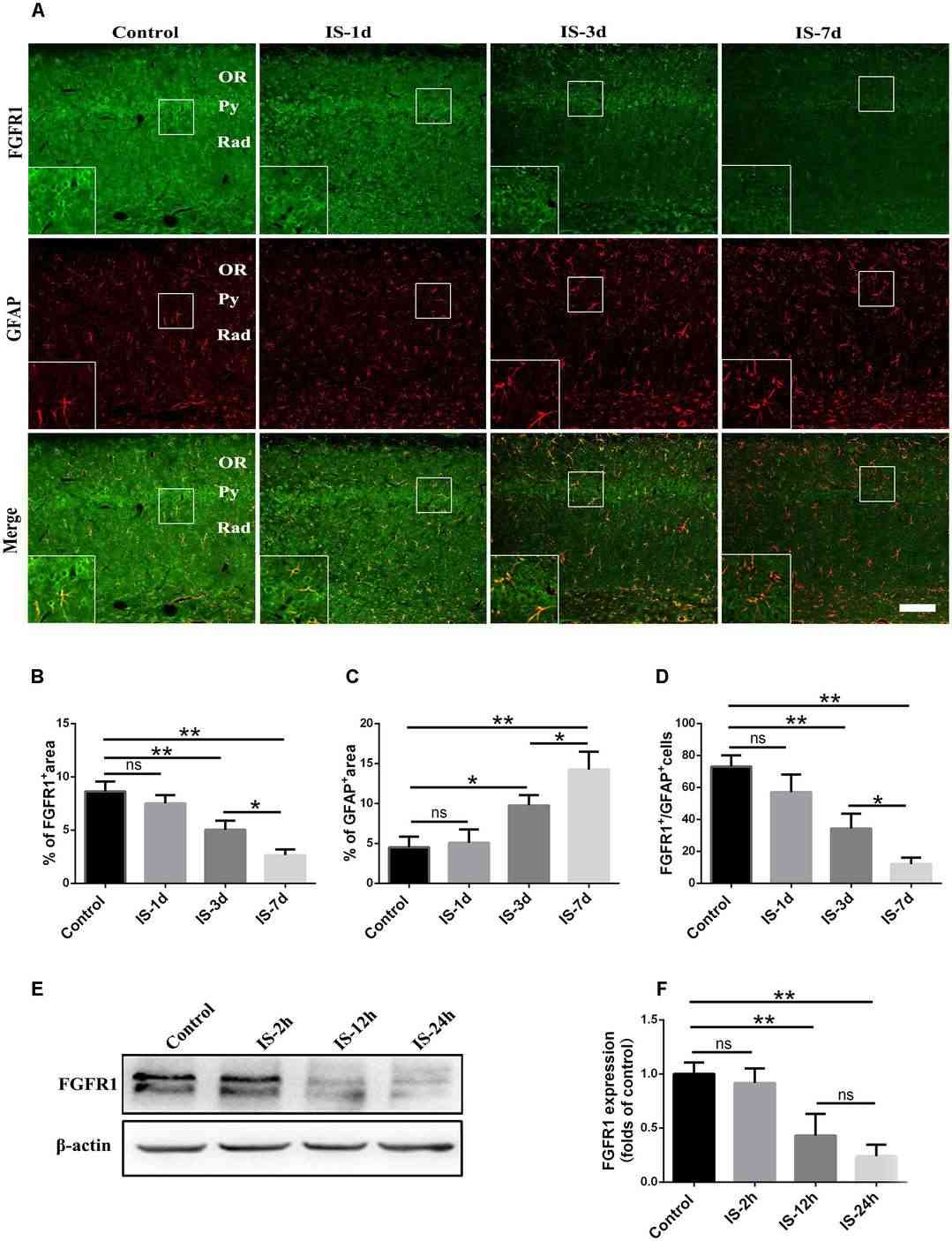 Fig. 2. Changes in the expression of FGFR1 after infrasound exposure (Shi J, Shi M, et al., 2018).
Fig. 2. Changes in the expression of FGFR1 after infrasound exposure (Shi J, Shi M, et al., 2018).
Hypersensitivity of the Vimentin Cytoskeleton to Net-Charge States and Coulomb Repulsion
The vimentin cytoskeleton, a vital structural component in mammalian cells, rapidly disassembles under hypotonic stress. This behavior suggests unrecognized dynamics in cellular structural proteins. Unger's team employed advanced microscopy techniques to observe vimentin behavior under changing ionic strength and pH, and manipulates vimentin charge properties.
They found that vimentin's stability is influenced by net negative charges causing Coulomb repulsion. Unlike vimentin, cytokeratin does not rapidly disassemble due to its mixed charge composition, indicating charge plays a key role in filament behavior under stress. They comparison cytokeratin with GFAP to further generalizes the net-charge mechanism of intermediate-filament intracellular stability. Examining PtK2 cells, endogenous vimentin and keratin coexist. After a 5 minute hypotonic treatment, cytokeratin retains its filament structure (Fig. 3A i), while vimentin fully disassembles (Fig. 3A ii,iii), as shown by STORM (Fig. 3A iv). Two-color live-cell imaging indicates that vimentin and keratin partially colocalize initially (Fig. 3B i). Under hypotonic conditions, vimentin disassembles within ~2 minutes (Fig. 3B ii) and fully diffuses by 4 minutes (Fig. 3B iii). Keratin shows no major changes, although some filaments are dislocated (Fig. 3B). In astrocytes, GFAP polymerizes into intermediate filaments in a fashion analogous to vimentin. With primary rat hippocampus astrocytes, they thus showed full disassembly of the endogenous GFAP cytoskeleton under hypotonic stresses, with STORM super-resolution microscopy resolving no remaining filaments (Fig. 3C).
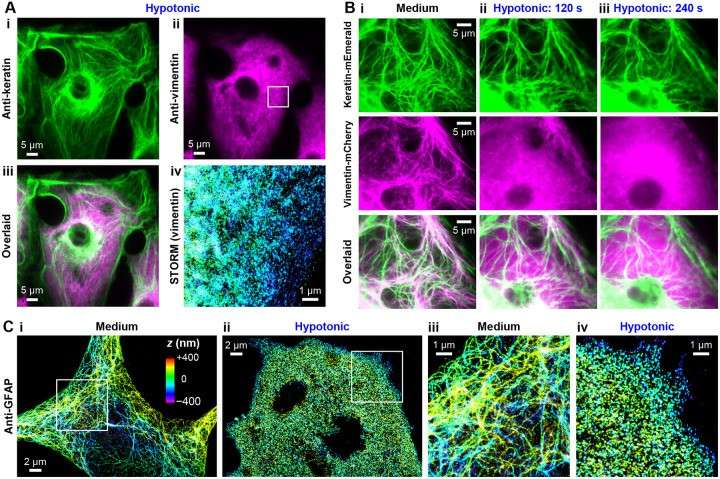 Fig. 3. Comparison with cytokeratin and GFAP further generalizes the net-charge mechanism of intermediate-filament intracellular stability (Unger BA, Wu CY, et al., 2024).
Fig. 3. Comparison with cytokeratin and GFAP further generalizes the net-charge mechanism of intermediate-filament intracellular stability (Unger BA, Wu CY, et al., 2024).
Ask a Question
Write your own review
- You May Also Need