ONLINE INQUIRY
Mouse Pulmonary Microvascular Endothelial Cells
Cat.No.: CSC-C9362W
Species: Mouse
Source: Lung
Morphology: Polygonal
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Mouse pulmonary microvascular endothelial cells (mPMVECs) are found in the microvasculature of lung tissue, and they are the main cell population that makes up the walls of pulmonary micro-vessels. They build up the alveolar-capillary barrier. These cells are spindle-shaped or polygonal, and have a cobblestone or pavement pattern when monolayers form, typical of endothelial morphology. The semipermeable membrane formed by mPMVECs plays an essential role in pulmonary gas exchange and the regulation of fluid and solute exchange between blood and lung interstitium. They are also involved in physiological regeneration, growth, wound healing, and inflammation. In addition, mPMVECs express a variety of markers, such as CD31 and Vimentin, which helps establish their endothelial identity.
In chronic pulmonary disorders such as acute respiratory distress syndrome (ARDS), pneumonia and pulmonary edema, mPMVECs are probably deficient in their barrier function. MPMVECs, as a result, are common targets in the study of lung diseases, including ARDS, COPD, and pulmonary hypertension. In patients with COPD, for example, injecting EVs from MPMVECs significantly reduces lung pathology and inflammation. MPMVECs are also used to study repair mechanisms in response to lung injury and angiogenesis.
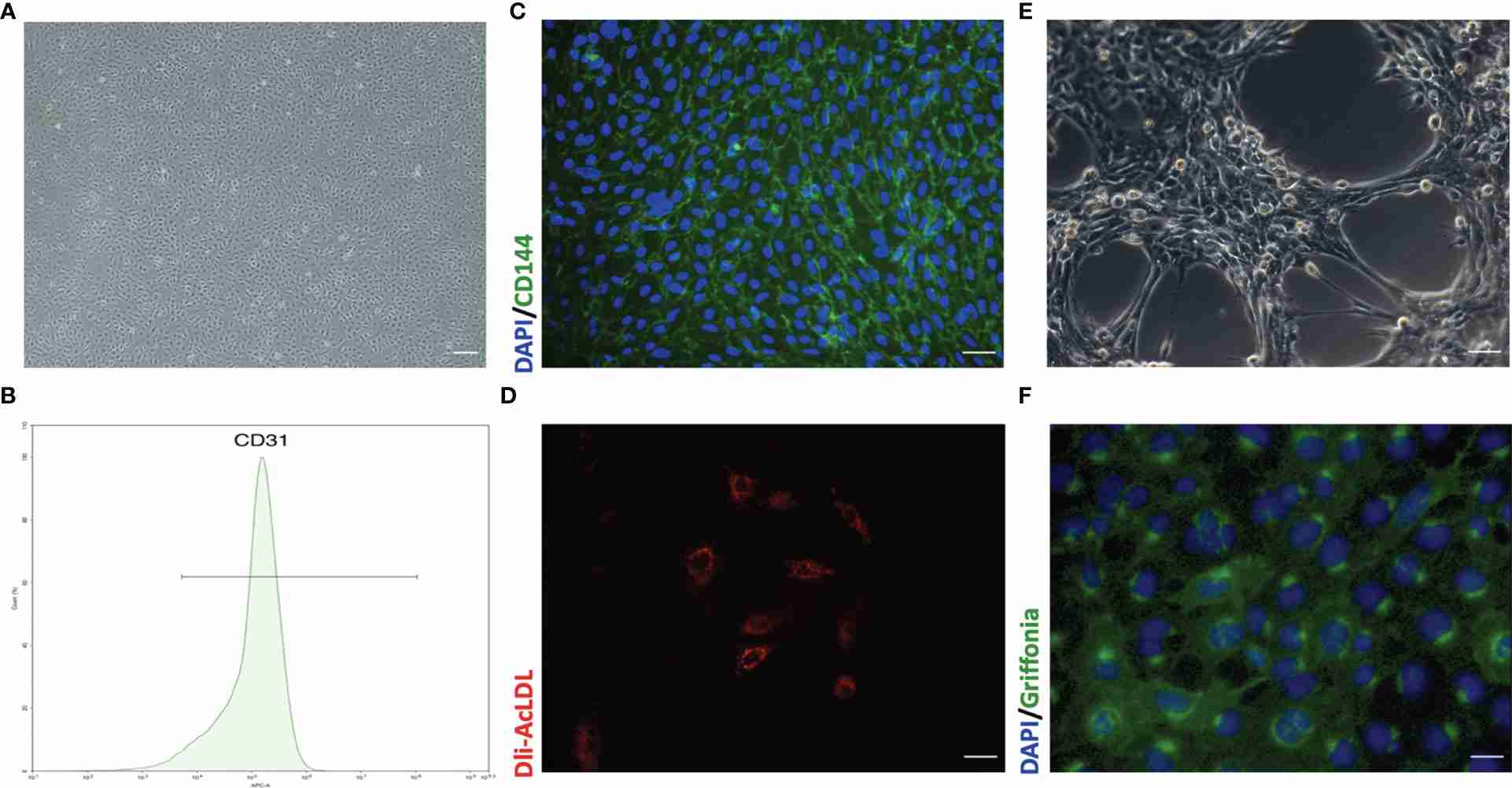 Fig. 1. Representative morphology and identification of primary mouse pulmonary microvascular endothelial cells (pMPMECs) (Liu X, Xia F, et al., 2021).
Fig. 1. Representative morphology and identification of primary mouse pulmonary microvascular endothelial cells (pMPMECs) (Liu X, Xia F, et al., 2021).
TRPM2 Knockdown Reduces Intracellular ROS Production and DNA Damage Induced by H9N2 Virus Infection
Influenza virus-related deaths from pneumonia are chiefly due to ALI or ARDS, where oxidative stress notably exacerbates viral pathogenesis. Pulmonary microvascular endothelial cells (PMVECs), critical for maintaining pulmonary microvascular integrity, suffer significant injury during infection. Transient receptor potential melastatin 2 (TRPM2). channels, responsive to oxidative stress, influence ROS production and endothelial function, marking them potential contributors to PMVEC damage due to viral interactions.
Wang's team explored the role of TRPM2 in H9N2 influenza virus infection on PMVECs, assessing whether inhibiting TRPM2 can mitigate ROS production, DNA damage, apoptosis, and alter inflammatory activities. In influenza infections, ROS is generated due to redox imbalance. To assess intracellular ROS levels caused by influenza virus, PMVECs with control shRNA and TRPM2 shRNA were both infected with A/Swine/Hebei/012/2008/(H9N2) at an MOI of 5, and measured at 12 hours post-infection. The H9N2 virus significantly increased ROS levels in control cells (Fig. 1), whereas TRPM2 knockdown notably reduced ROS production in infected PMVECs. This demonstrates that TRPM2 knockdown effectively decreases ROS production induced by H9N2 infection. For assessing DNA damage, control cells were infected under the same conditions and stained with anti-phospho-histone H2A.X and anti-phospho-ATM antibodies. Analyzed using the Muse Cell Analyzer, results showed that TRPM2 knockdown significantly reduced H9N2-induced DNA damage (Fig. 2). The above results showed that inhibition of TRPM2 channels reduced H9N2 virus-induced intracellular ROS production, decreased DNA damage.
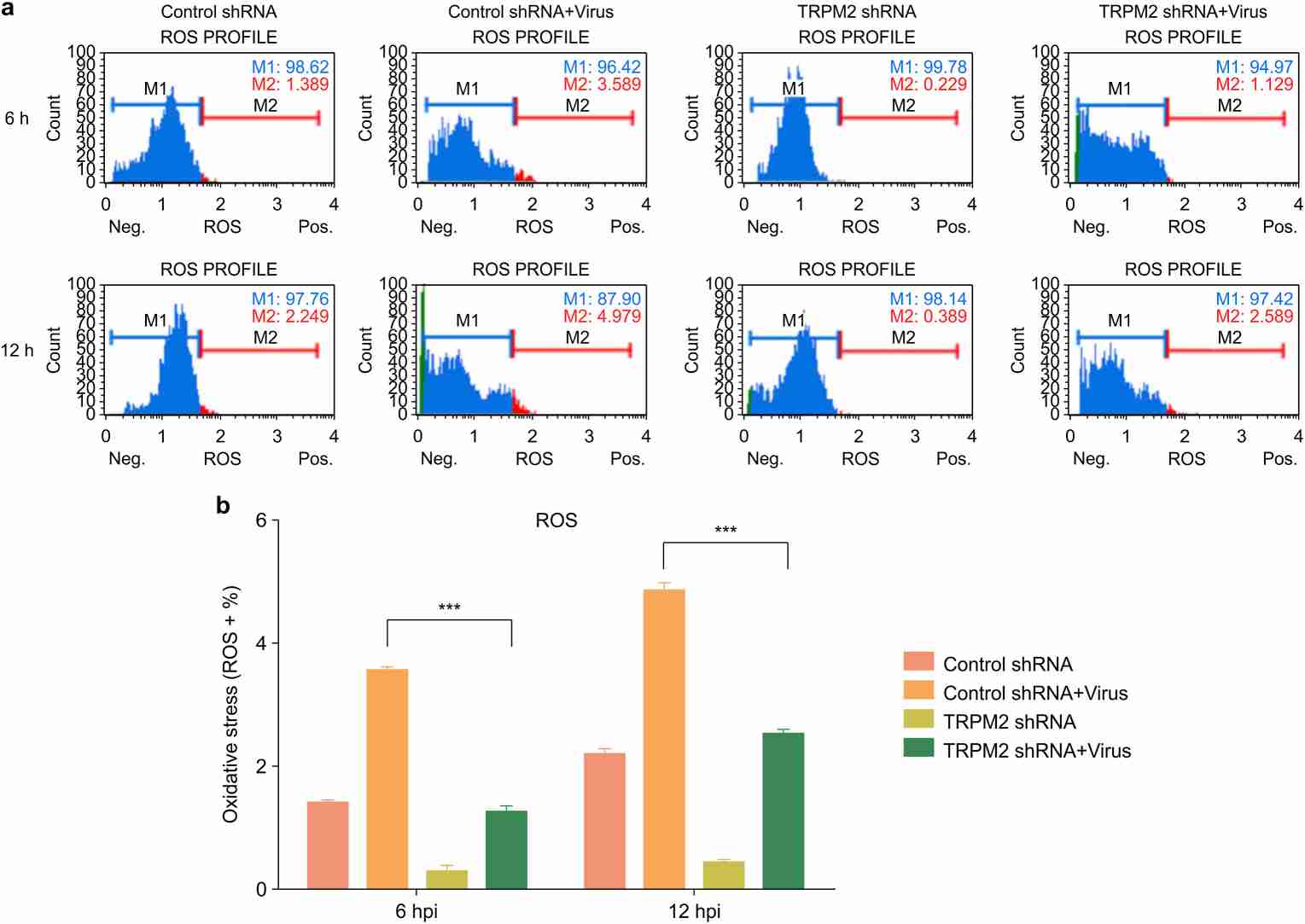 Fig. 1. TRPM2 knockdown attenuated intracellular ROS production induced by the H9N2 virus (Wang S, Liang T, et al., 2020).
Fig. 1. TRPM2 knockdown attenuated intracellular ROS production induced by the H9N2 virus (Wang S, Liang T, et al., 2020).
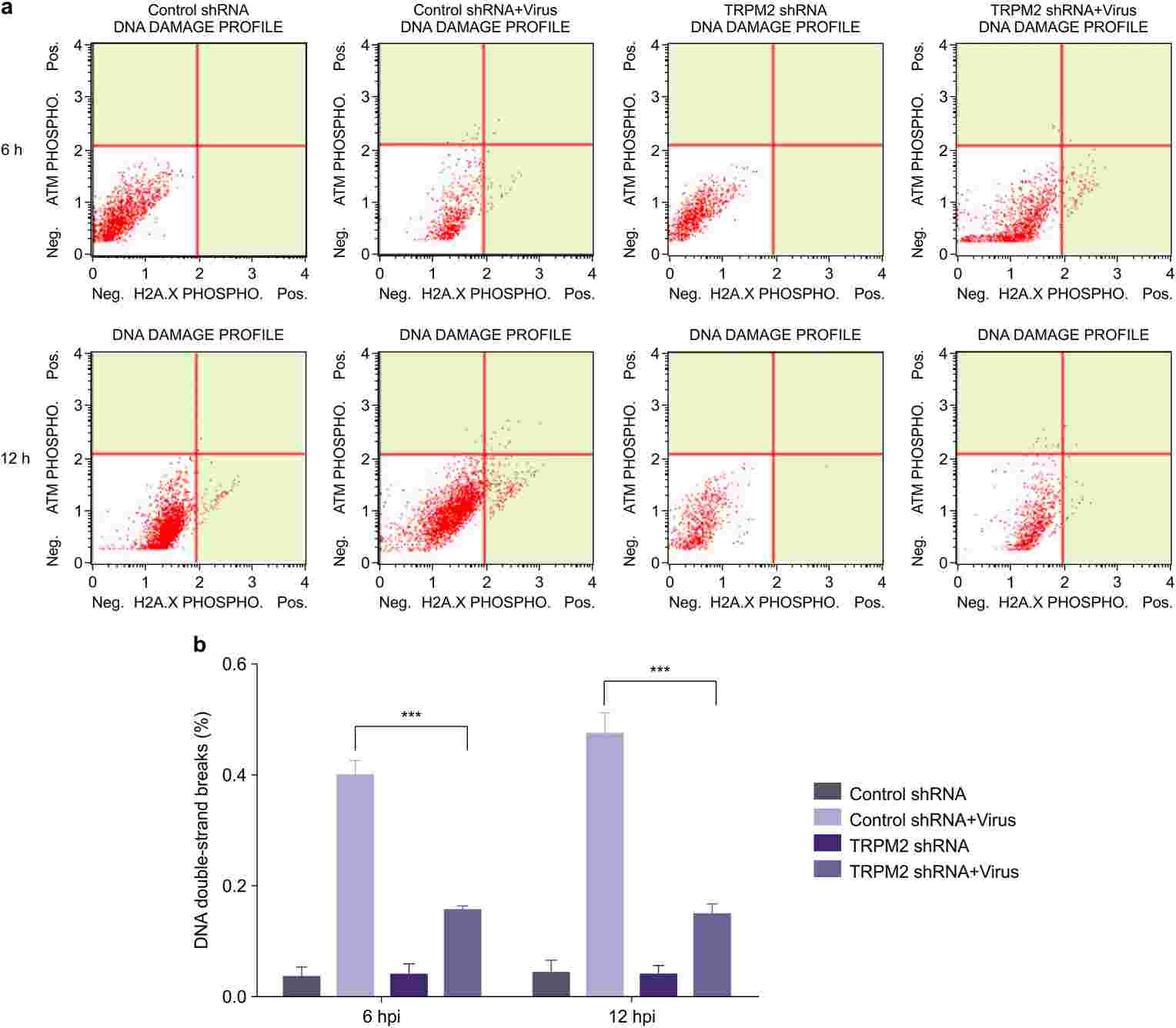 Fig. 2. TRPM2 knockdown decreased DNA damage induced by H9N2 viral infection (Wang S, Liang T, et al., 2020).
Fig. 2. TRPM2 knockdown decreased DNA damage induced by H9N2 viral infection (Wang S, Liang T, et al., 2020).
CircPalm2 is a Stable circRNA and is Increased by LPS Treatment
Sepsis-induced acute lung injury (sepsis-ALI) is a severe condition characterized by an excessive inflammatory immune response leading to potential irreversible pulmonary damage and high mortality. Recent findings have indicated the involvement of circular RNAs (circRNAs) in the regulation of complex pathologies related to sepsis-ALI. CircPalm2, among such circRNAs, has been identified as highly expressed in septic conditions.
To investigate the clinical value of circPalm2 in sepsis-ALI, primary murine pulmonary microvascular endothelial cells (MPVECs) were exposed LPS to imitate the injury of cells in sepsis-ALI in vitro. CCK-8 assay showed that LPS treatment reduced MPVEC viability dose-dependently at 50 or 100 ng/mL after 24 and 48 hours. Thus, 100 ng/mL was used for further analysis. After 24 hours of exposure, qRT-PCR results showed that circPalm2 expression was significantly up-regulated (Fig. 3A). RNase R digestion in MPVECs confirmed that while RNase R quickly degraded linear Palm2 mRNA, it did not affect circPalm2, demonstrating its round structure (Fig. 3B). Subcellular localization analysis revealed circPalm2 primarily in the cytoplasm of MPVECs (Fig. 3C). This suggests circPalm2 might act as a miRNA sponge and its expression changes could be linked to sepsis-ALI progression.
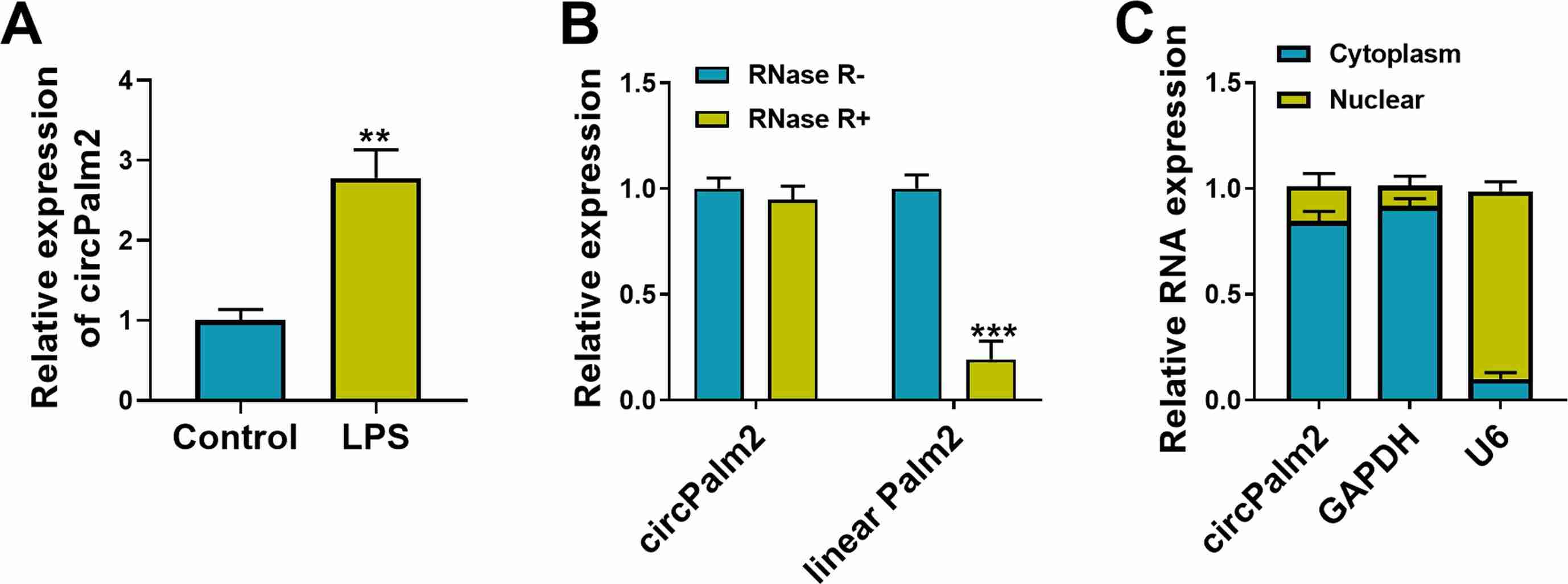 Fig. 3. CircPalm2 is a stable circRNA and is increased by LPS treatment (Mu Q, Zhang C, et al., 2022).
Fig. 3. CircPalm2 is a stable circRNA and is increased by LPS treatment (Mu Q, Zhang C, et al., 2022).
Ask a Question
Write your own review