ONLINE INQUIRY
Mouse Osteoblasts
Cat.No.: CSC-C9340W
Species: Mouse
Source: Bone
Morphology: Multipolar
Cell Type: Osteoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse osteoblasts typically come from bone tissue, especially calvarial bones and long bones (femur, tibia). Creative Bioarray's mouse osteoblasts are isolated from the normal mouse femoral bone tissue. They are generally found on the outside of bone tissues, such as the periosteum and endosteum. They are tightly integrated to the bone matrix and, in times of bone growth and repair, they produce and secrete various organic and inorganic molecules (collagen, osteocalcin, osteopontin) that form and remodel bone tissue. They also react to a variety of growth factors and hormones to regulate bone metabolism and homeostasis. For example, in growth plate areas on long bones, osteoblasts engage in the production of new bone, allowing continuous bone lengthening and thickening; when fractures heal, they flutter quickly to the damaged site to kick off the process of bone regeneration.
Mice osteoblast cell lines are widely employed for bone formation and metabolism. By studying osteoblasts' gene expression, signalling pathways and cell behavior across developmental levels, scientists can understand which regulatory mechanisms and molecular processes regulate bone development. Mouse osteoblasts have also been used in the investigation of several bone disorders, including osteoporosis and osteosclerosis. When it comes to bone tissue engineering, mouse osteoblasts are the primary seed cells for engineered bone tissues.
8-Nitro-cGMP Suppresses Mineralization by Mouse Osteoblasts
Earlier studies have shown that nitric oxide (NO) and reactive oxygen species (ROS) play a role in the bone-remodeling process to positively influence osteoclast differentiation and bone resorption. More recently, 8-nitro-cGMP, a second messenger of NO and ROS, was found to be responsible for osteoclastogenesis. In this paper, Kaneko's team investigated how 8-nitro-cGMP modulates osteoblastic phenotypes in mouse osteoblasts and observed that it suppresses osteoblast mineralisation.
8-nitro-cGMP can be sulfhydrated with hydrogen sulphide, or more effectively with cysteine hydropersulfide (CysSSH) and its reactive sulfur species (RSS), forming 8-SH-cGMP. 8-SH-cGMP activates cGMP-dependent kinase but it can't S-guanylate protein sulfhydryl groups. NO and ROS scavenge out its sulfhydryl group, and phosphodiesterases hydrolyse the resulting cGMP into GMP. RSS, and especially CysSSH, are key regulators of intracellular 8-nitro-cGMP metabolism. CysSSH synthase (CARS2/CPERS) is a mitochondrial cysteinyl-tRNA synthase that produces CysSSH from l-cysteine. They therefore explored the impact of knocking down the Cars2 gene (Fig. 1A) on osteoblastic phenotype expression in mouse osteoblasts grown with BMP-2 to clarify the function of endogenously produced 8-nitro-cGMP. These findings revealed that silencing Cars2 in mouse osteoblasts increases S-guanylated proteins and endocrine 8-nitro-cGMP (Fig. 1B), decreasing ALP activity (Fig. 1C and D), mineralisation, and downregulation of Bglap (Fig. 1E), suggesting both exogenous and endogenous suppression of osteoblastic expression via 8-nitro-cGMP.
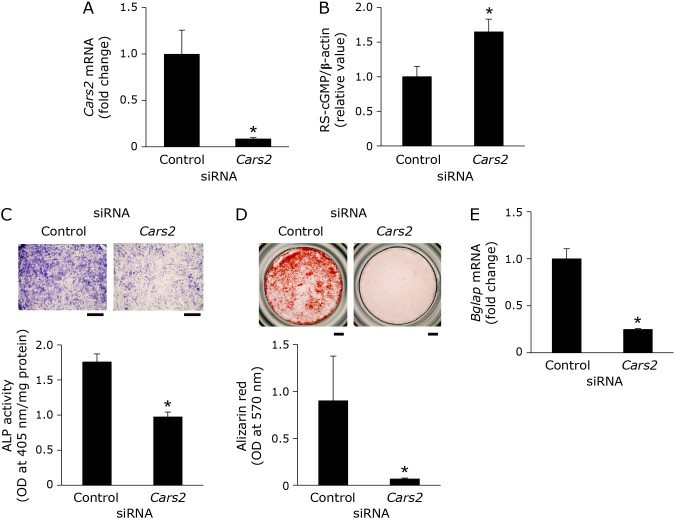 Fig. 1. Effects of Cars2 siRNA on osteoblastic phenotypes in mouse osteoblasts (Kaneko K, Miyamoto Y, et al., 2022).
Fig. 1. Effects of Cars2 siRNA on osteoblastic phenotypes in mouse osteoblasts (Kaneko K, Miyamoto Y, et al., 2022).
Simulated Microgravity Reduces LTCC Activity in Osteoblasts
Calcium homeostasis in osteoblasts is a critical feature of both health and disease. Mechanical stimulations stimulate intracellular free calcium in osteoblasts and drive osteogenesis and bone formation. But microgravity - the mechanical unloading mode - induces irreversible bone loss, notably through depletion of osteoblasts. How microgravity determines the amount of calcium in osteoblasts, and how it works, is far from clear.
Calcium-gated voltage-sensitive calcium channels, specifically, L-type voltage-sensitive calcium channels (LTCCs), that allow calcium to cross the plasma membrane from the outside of the body are osteoblasts' major regulators of intracellular calcium homeostasis. Sun's team used whole-cell and cell-attached patch-clamps with trypsinized cells to see how mock microgravity impacted LTCC expression in primary mouse osteoblasts. Whole-cell LTCC currents are lower under simulated microgravity (Fig. 2C) than under control (Fig. 2B). They detected upward currents peaking at +10 mV. Bay K8644 raised current level, but nifedipine almost totally killed it, suggesting Ba2+ currents in LTCCs. Currents, adjusted for membrane capacitance (Cm), were smaller in the microgravity group (Fig. 2B and 2C). LTCC current density was lower in the microgravity group (Fig. 2D), peak densities averaged − 3.15 ± 0.23 versus − 5.43 ± 0.45 pA/pF (Fig. 2D). There was no significant voltage difference between the activation and inactivation curves (Fig. 2E and 2F). Cell-attached patches revealed similar findings (Fig. 3A-F), with decreased NPO in microgravity but unchanged unitary conductance. Simulated microgravity also reduced calcium inflow from the extracellular space.
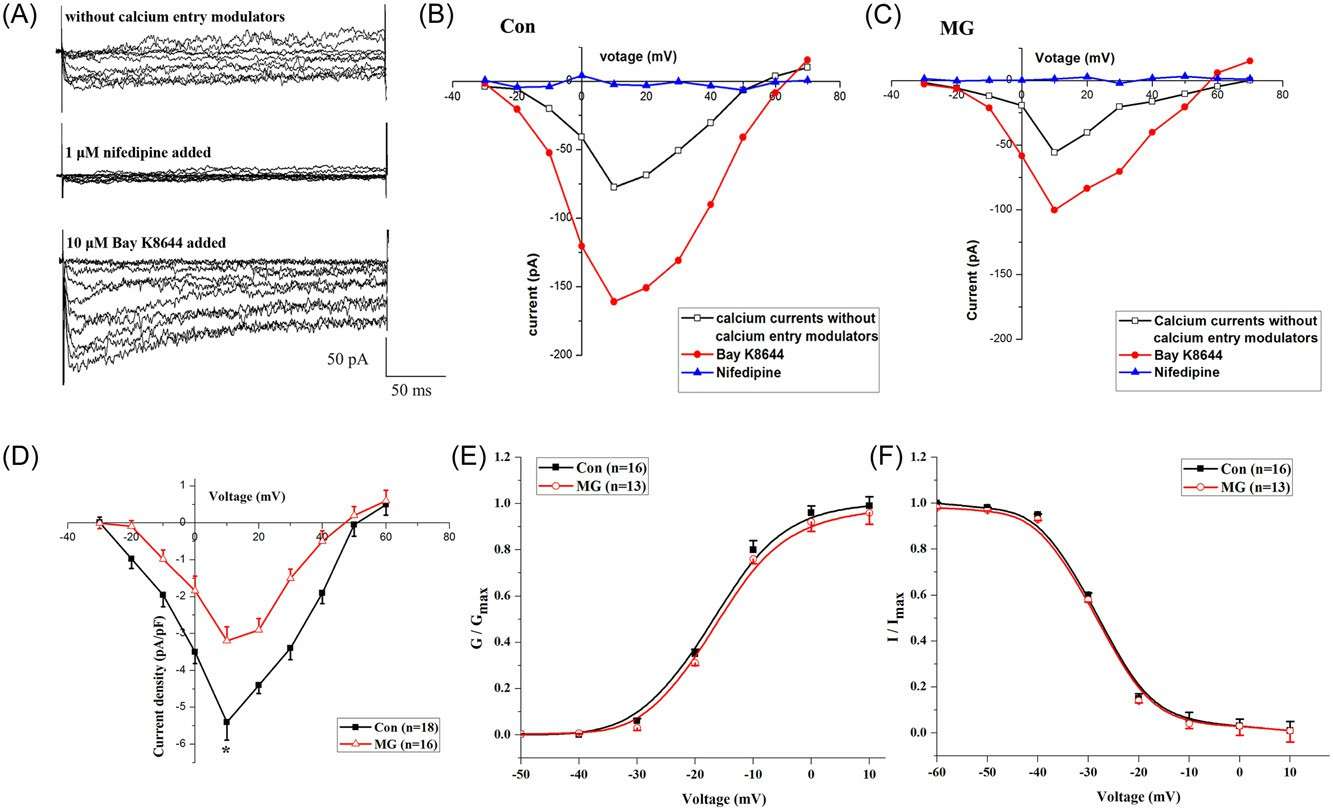 Fig. 2. Whole-cell LTCC currents in primary mouse osteoblasts from the Con and MG groups using patch-clamp techniques (Sun Z, Li Y, et al., 2019).
Fig. 2. Whole-cell LTCC currents in primary mouse osteoblasts from the Con and MG groups using patch-clamp techniques (Sun Z, Li Y, et al., 2019).
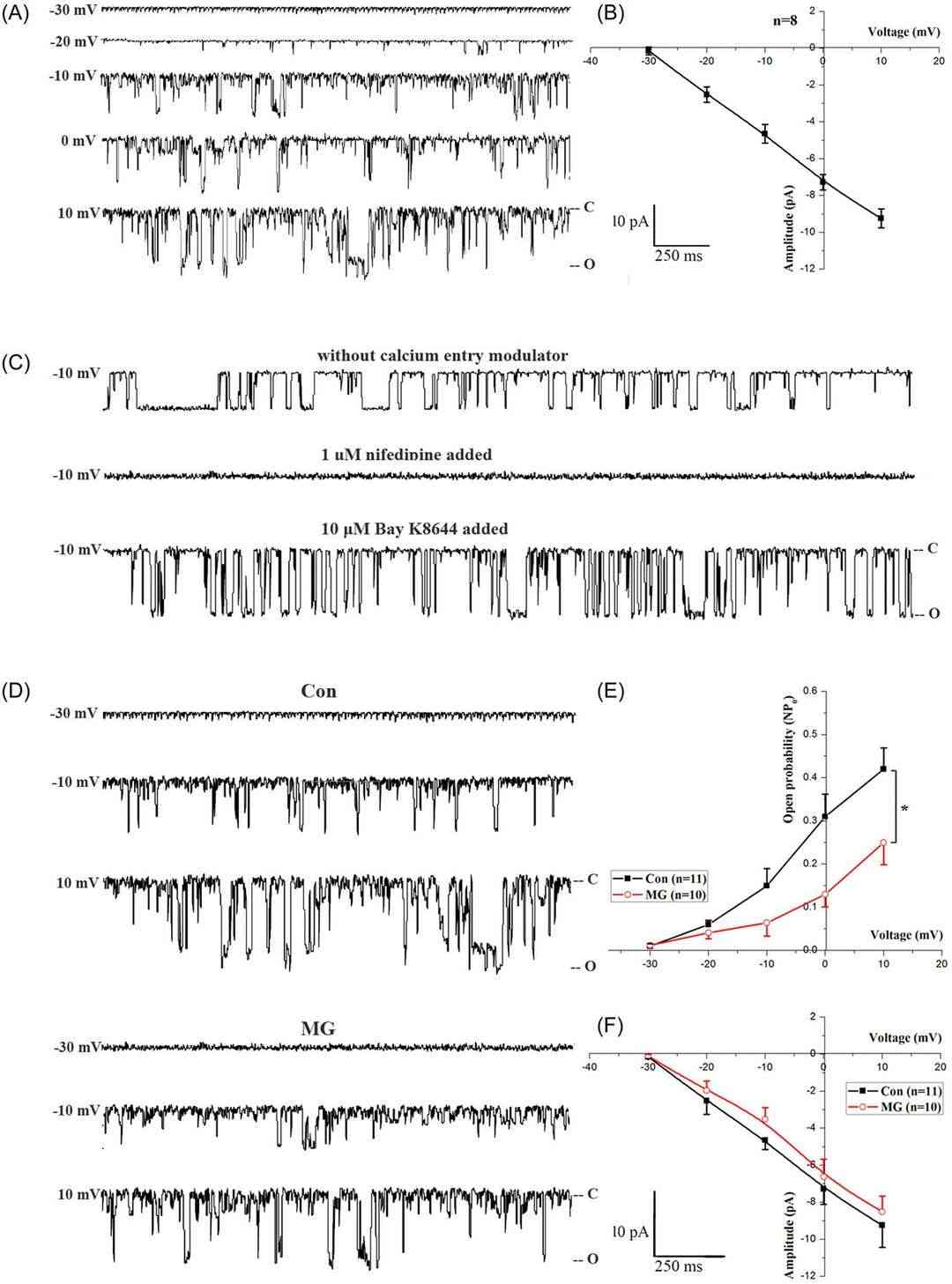 Fig. 3. Effects of MG on LTCC single-channel activity from cell-attached patch membranes in primary mouse osteoblasts (Sun Z, Li Y, et al., 2019).
Fig. 3. Effects of MG on LTCC single-channel activity from cell-attached patch membranes in primary mouse osteoblasts (Sun Z, Li Y, et al., 2019).
Ask a Question
Write your own review