ONLINE INQUIRY

Human Vein Endothelial Cells
Cat.No.: CSC-C8605W
Species: Human
Source: Vein
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human vein endothelial cells (HVECs) originate mostly from the venous blood vessels of the body like the umbilical vein and the great saphenous vein. Of them, human umbilical vein endothelial cells (HUVECs) from newborn umbilical veins are the most commonly used research model. These cells are "cobblestone-like" in appearance and they express a variety of endothelial cell markers such as CD31, von Willebrand Factor (vWF), and CD34.
As an endothelial cell type, HUVECs have a host of physiological roles including maintaining the wall of the vascular vessel, angiogenesis and healing, controlling the permeability of the artery and initiating inflammation. All these functions make HUVECs invaluable for research from cardiovascular disease to tumor angiogenesis to inflammatory disorders. In particular, HUVECs are used extensively to study angiogenesis and disease-associated angiogenic processes. HUVECs also mimic endothelial cells in an inflammatory milieu, making them attractive for studying inflammatory disorders like atherosclerosis and diabetic vasculopathy. Since they are so sensitive to many different drugs, HUVECs are also widely employed in drug screens and toxicology analyses. HUVECs can also be co-cultured with Mesenchymal Stem Cells (MSCs) to produce vascularized tissue engineering models. In cancer research, HUVECs are chiefly utilized to investigate angiogenesis in solid tumors, uncovering mechanisms and seeking inhibitors of tumor vascularization.
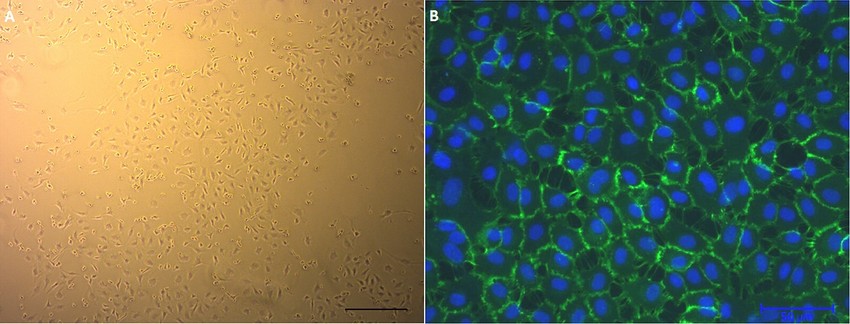 Fig. 1. Human umbilical vein endothelial cells (HUVECs) in culture. (A) Phase-contrast micrograph. (B) Cell surface marker CD31 (green) (Ertan A B, Kenar H, et al., 2017).
Fig. 1. Human umbilical vein endothelial cells (HUVECs) in culture. (A) Phase-contrast micrograph. (B) Cell surface marker CD31 (green) (Ertan A B, Kenar H, et al., 2017).
PPAP with Protective Effect on Human Vein Endothelial Cells Injured by High-Glucose
Originating from Hypericum acmosepalum, polycyclic polyprenylated acylphloroglucinol (PPAP) are highly oxygenated compounds with significant pharmacological effects, such as anti-inflammatory and antioxidant activities. These compounds, crucial for treating endothelial damage due to hyperglycemia in diabetes.
To explore the potential against high glucose injury compounds from H. acmosepalum, the EtOH extract of the aerial parts of H. acmosepalum was fractionated chromategraphically to yield sixteen PPAPs (1–16). Then, a bioassay assessed the protective effects of isolated PPAPs on human umbilical vein endothelial cells (HUVECs) damaged by high glucose. Initially, the cytotoxicity of compounds 1–4, and 7–16 was measured using the CCK-8 assay, with hyperacmotone C showing cytotoxicity at an IC50 of 12.9 μg/mL. The compounds' protective effects at 50 μg/mL were further evaluated using the CCK-8 assay (Fig. 1). For compounds with activity surpassing aspirin by 33.0%, six concentration gradients were tested to find the EC50. Compound 13 displayed a promising EC50 of 4.1 μg/mL, indicating significant protection of HUVECs. Its cell viability surpassed the high-glucose injured model group, suggesting repair capabilities. Additionally, a β-galactosidase assay showed that compound 13 reduced cell senescence, as evidenced by decreased positive staining (Fig. 2). These results highlight compound 13's potential anti-hyperglycemia effect.
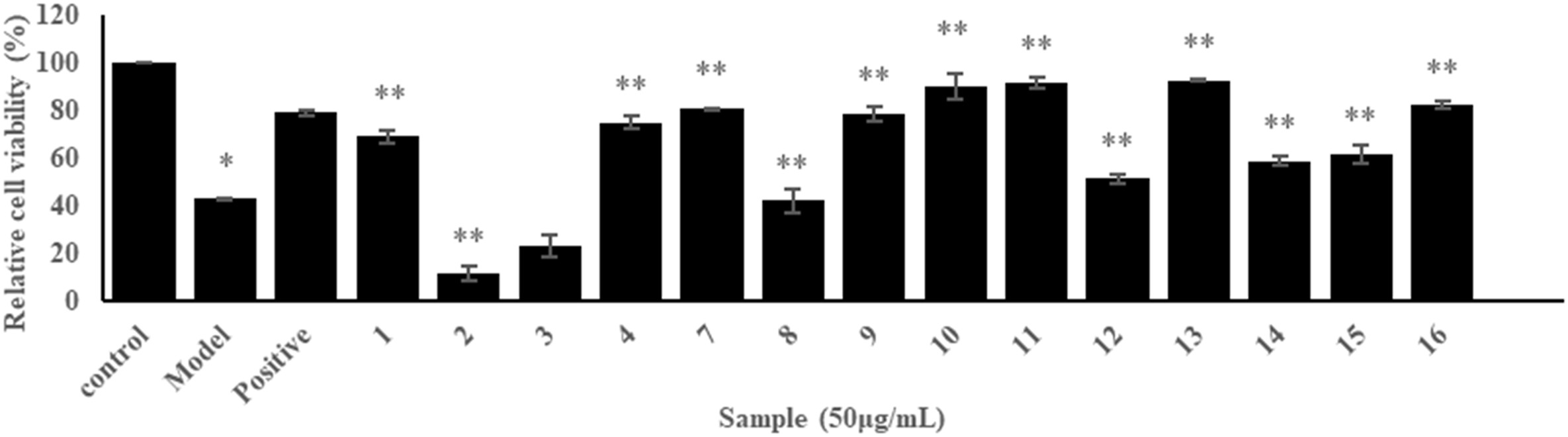 Fig. 1. Screening results of protective effect of compounds 1–4, and 7–16 at 50 μg/mL on HUVECs injured by high-glucose (Wang A, Han H, et al., 2023).
Fig. 1. Screening results of protective effect of compounds 1–4, and 7–16 at 50 μg/mL on HUVECs injured by high-glucose (Wang A, Han H, et al., 2023).
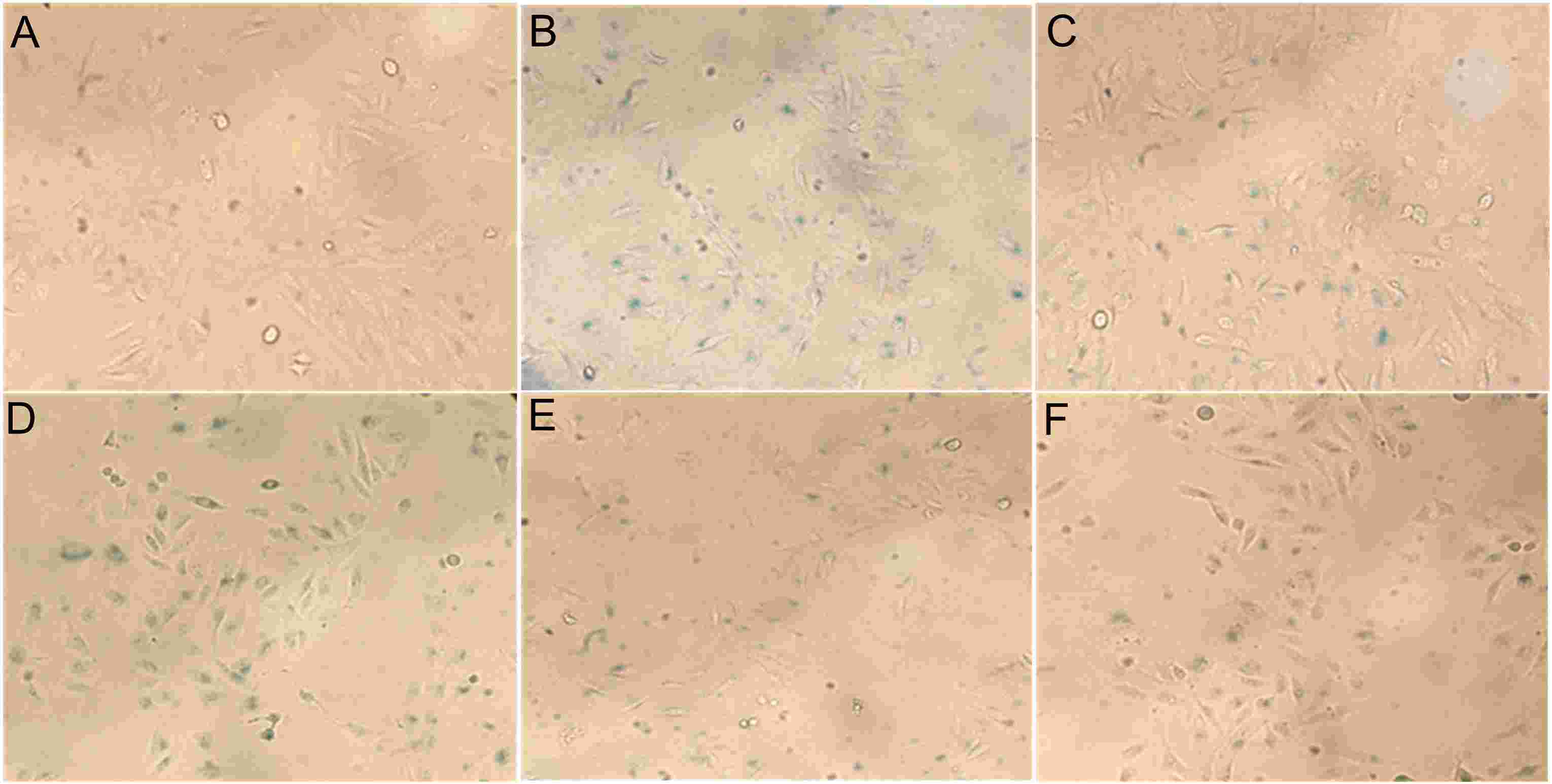 Fig. 2. The HUVECs morphologies of Control (A), Model (B), Positive Control (C), and sample groups at 1 (D), 5 (E), 50 (F) μg/mL of compound 13 (Wang A, Han H, et al., 2023).
Fig. 2. The HUVECs morphologies of Control (A), Model (B), Positive Control (C), and sample groups at 1 (D), 5 (E), 50 (F) μg/mL of compound 13 (Wang A, Han H, et al., 2023).
ALA Improves the Viability of H2O2-Induced HUVECs and Reduces LDH Release
Atherosclerosis arises from oxidative stress and inflammatory processes affecting vascular endothelial cells. ROS-mediated apoptosis and inflammatory responses are key contributors. Alpha-lipoic acid (ALA), a potent antioxidant, may mitigate these effects. Wang et al. utilized an H2O2-induced human umbilical vein endothelial cells (HUVECs) injury model to evaluate ALA's protective mechanism by administering ALA with various concentrations.
The selected experimental concentrations for the low, medium and high-dose ALA groups were 100, 200 and 400 μmol/L, respectively. After incubating HUVECs with 250 μmol/L H2O2 and varying ALA concentrations for 48 hours, an MTT assay evaluated cell viability. Figure 3(b) shows that the cell survival rate of the H2O2 injury group significantly dropped compared to the control. ALA co-incubation increased survival rates to 57.09%, 68.17%, and 78.10%, with significant differences among doses, confirming ALA's protective effect (Fig. 3b). LDH is considered to be a biomarker for assessing cellular injury. As expected, the model group exhibited higher LDH activity than controls. ALA reduced LDH release in a dose-dependent manner (Fig. 4).
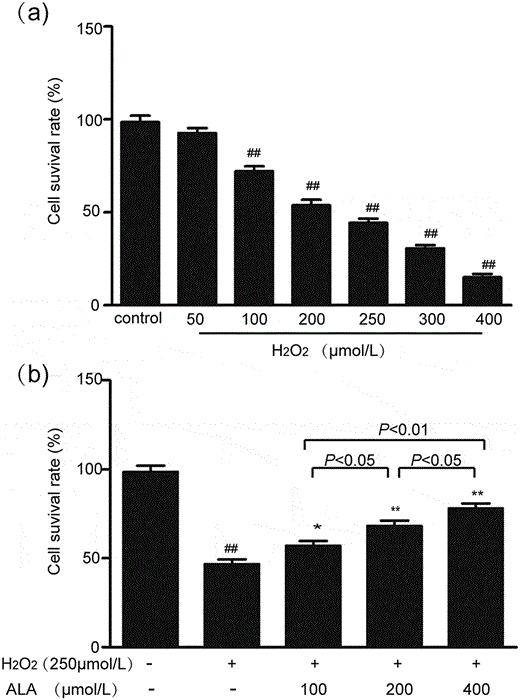 Fig. 3. Effects of H2O2 and ALA on HUVECs cell viability (Wang W, An L, et al., 2020).
Fig. 3. Effects of H2O2 and ALA on HUVECs cell viability (Wang W, An L, et al., 2020).
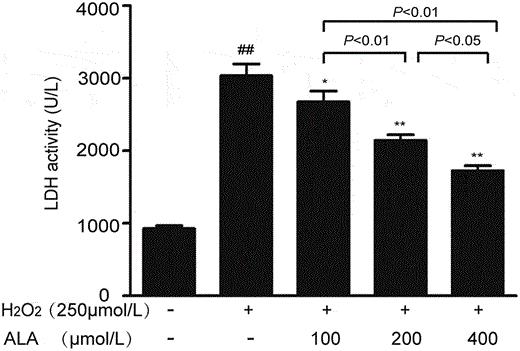 Fig. 4. Effects of different concentrations of ALA on the LDH activity in H2O2-induced HUVECs (Wang W, An L, et al., 2020).
Fig. 4. Effects of different concentrations of ALA on the LDH activity in H2O2-induced HUVECs (Wang W, An L, et al., 2020).
Ask a Question
Write your own review


