ONLINE INQUIRY

Human Thyrocyte Cells
Cat.No.: CSC-C9391W
Species: Human
Source: Thyroid
Morphology: Polygonal
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human thyrocytes cells originate mainly from thyroid gland material. The thyroid gland serves as a vital part of the human endocrine system and sits at the front of the neck consisting of follicular cells known as thyrocytes. A gel-like material mainly consisting of thyroglobulin occupies the follicular cavity where it functions as a precursor to thyroid hormones. Thyroid cells inhabit these follicles and demonstrate a round to oval shape with a diameter ranging between 20 and 30 micrometers. The cells display standard endocrine cell characteristics through their significant rough endoplasmic reticulum and Golgi apparatus which enable protein synthesis and secretion mainly of thyroid hormones like triiodothyronine (T3) and thyroxine (T4). The hormones control normal bodily processes through their regulation of metabolism and energy balance together with growth and development. Thyroid follicular epithelial cells perform essential roles in iodine uptake and transport to facilitate thyroid hormone production. Immunofluorescence staining reveals thyroid cell morphology through detection of markers like the sodium/iodide symporter (NIS), thyroid peroxidase (TPO), and thyroglobulin (Tg).
Scientists frequently use Human Thyrocyte Cells to understand how thyroid diseases develop. By creating models that mimic Graves' disease or autoimmune thyroiditis (AIT) researchers can study TSH receptor antibodies and their impact on thyroid cells.
Ferruginol Inhibited the Proliferation and Induced Apoptotic Cell Death of Thyroid Cancer Cells
Thyroid cancer is a major cause of mortality and morbidity worldwide, with a pressing need for effective chemotherapy due to a lack of biomarkers and side effects of current treatments. Ferruginol, a plant-based compound, is studied for thyroid cancer treatment due to its success with prostate and non-small cell lung cancers. Luo's team evaluated ferruginol's anticancer effects specifically on MDA-T32 thyroid cancer cells.
The MTT assay tested ferruginol's impact on MDA-T32 and normal human thyrocyte cells at concentrations from 0 to 160 μM. Spectrophotometer readings at 570 nm revealed ferruginol suppressed MDA-T32 cell growth dose-dependently, with an IC50 of 12 μM, while its effect on normal thyrocyte cells was milder, having an IC50 of 92 μM (Fig. 1A). To explore the antiproliferative mechanism, MDA-T32 cells treated with ferruginol were stained with AO/EB and DAPI. AO/EB staining showed increased orange cells with higher ferruginol concentrations (Fig. 1B), and DAPI staining showed more cells with white nuclei, indicating apoptosis (Fig. 1C). Annexin V/PI staining revealed untreated samples had 5.6% apoptotic cells, increasing to about 61% at 24 μM, demonstrating dose-dependent apoptosis (Fig. 1D).
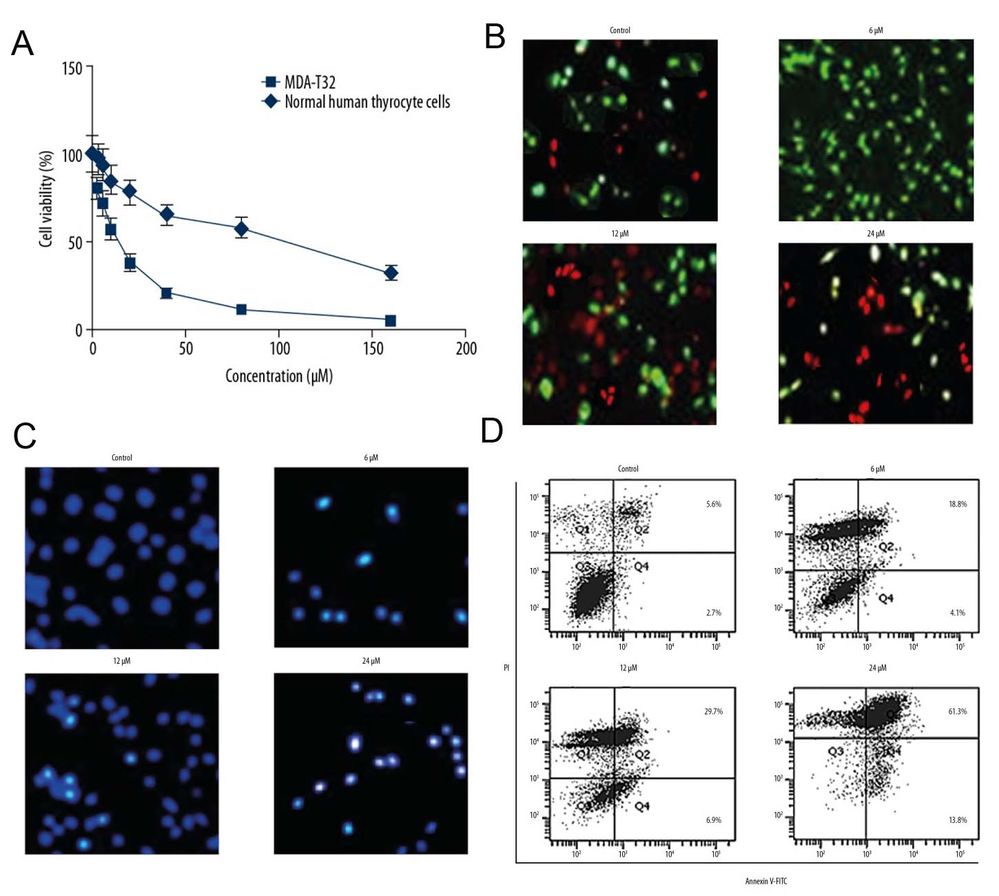 Fig. 1. (A) Ferruginol inhibited the proliferation of MDA-T32thyroid cancer cells. (B-D) Ferruginol induced apoptotic cell death of MDA-T32 cells (Luo G, Zhou J, et al., 2019).
Fig. 1. (A) Ferruginol inhibited the proliferation of MDA-T32thyroid cancer cells. (B-D) Ferruginol induced apoptotic cell death of MDA-T32 cells (Luo G, Zhou J, et al., 2019).
Primary Human Thyrocytes Expressed Thyroid-Specific Genes at Higher Levels in the Transwell System
The thyroid is vital for hormone production, specifically T3 and T4, essential for growth and metabolic regulation. Thyroid dysfunctions are prevalent endocrine disorders. Traditionally, thyroid cell lines, while maintaining gene expression, don't produce hormones in vitro, and primary thyrocytes face challenges such as loss of functional structure and interference from exogenous hormones. Jiang's team developed a culture system that sustains the functionality of thyrocytes to produce and secrete thyroid hormones in vitro.
To retain primary thyrocyte polarity in vitro, a Transwell system was used. Human thyrocytes were plated onto a collagen-coated porous membrane in the inner chamber. When cells reached full confluency, DMEM was added to the inner chamber, with DMEM and 10% FBS in the outer chamber, mimicking lumen and capillary. This setup allowed each thyrocyte surface to access different medium components. In contrast, conventional monolayer cultures expose only one surface to DMEM with 10% FBS. Thyroid-specific gene expressions in both culture systems were compared with donor tissues. Thyroid-functional and transcriptional genes showed reduced expression in both systems compared to donor tissues (Fig. 2). However, Transwell system maintained higher gene expression levels (30-80%) than monolayer (1-20%) (Fig. 2). Immunofluorescence showed TG distribution was more extensive in Transwell, with NIS staining only evident in Transwell, suggesting its superiority over conventional culture.
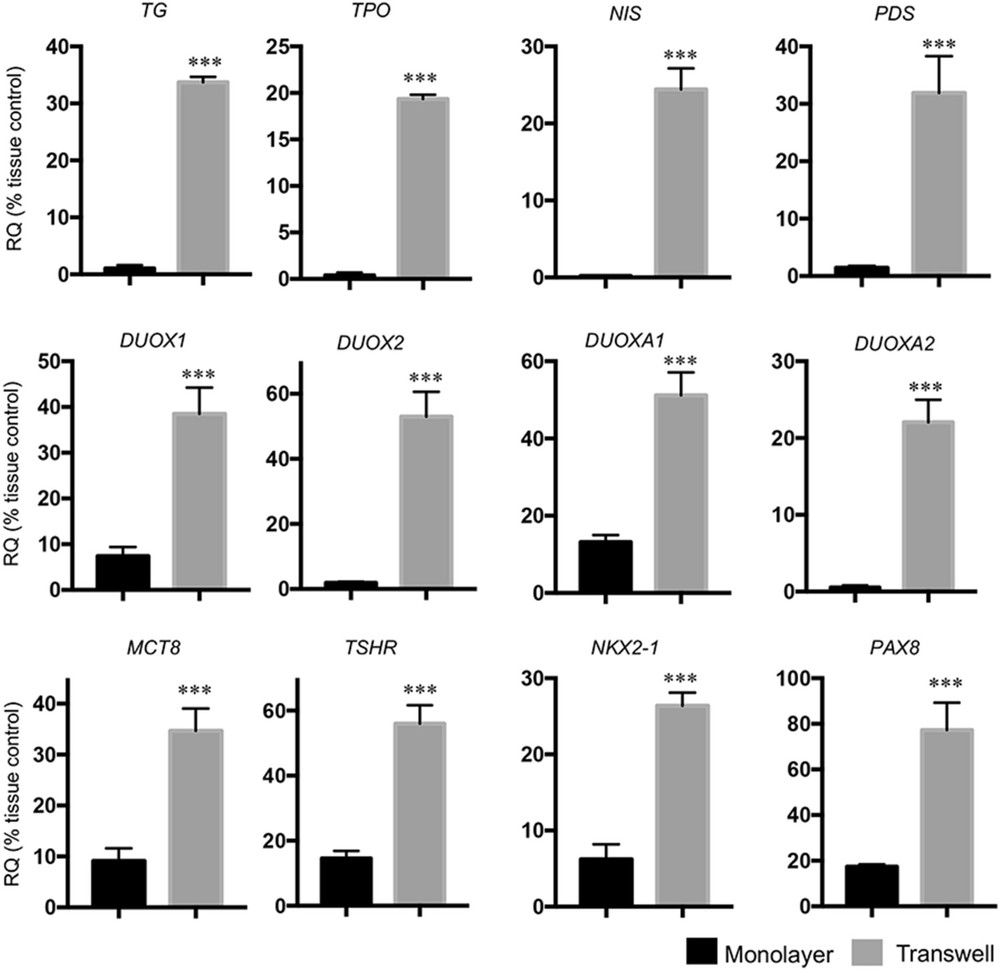 Fig. 2. The expression levels of thyroid-specific genes in primary human thyrocytes were higher in the Transwell system than that in the monolayer culture (Jiang B, Wang C, et al., 2023).
Fig. 2. The expression levels of thyroid-specific genes in primary human thyrocytes were higher in the Transwell system than that in the monolayer culture (Jiang B, Wang C, et al., 2023).
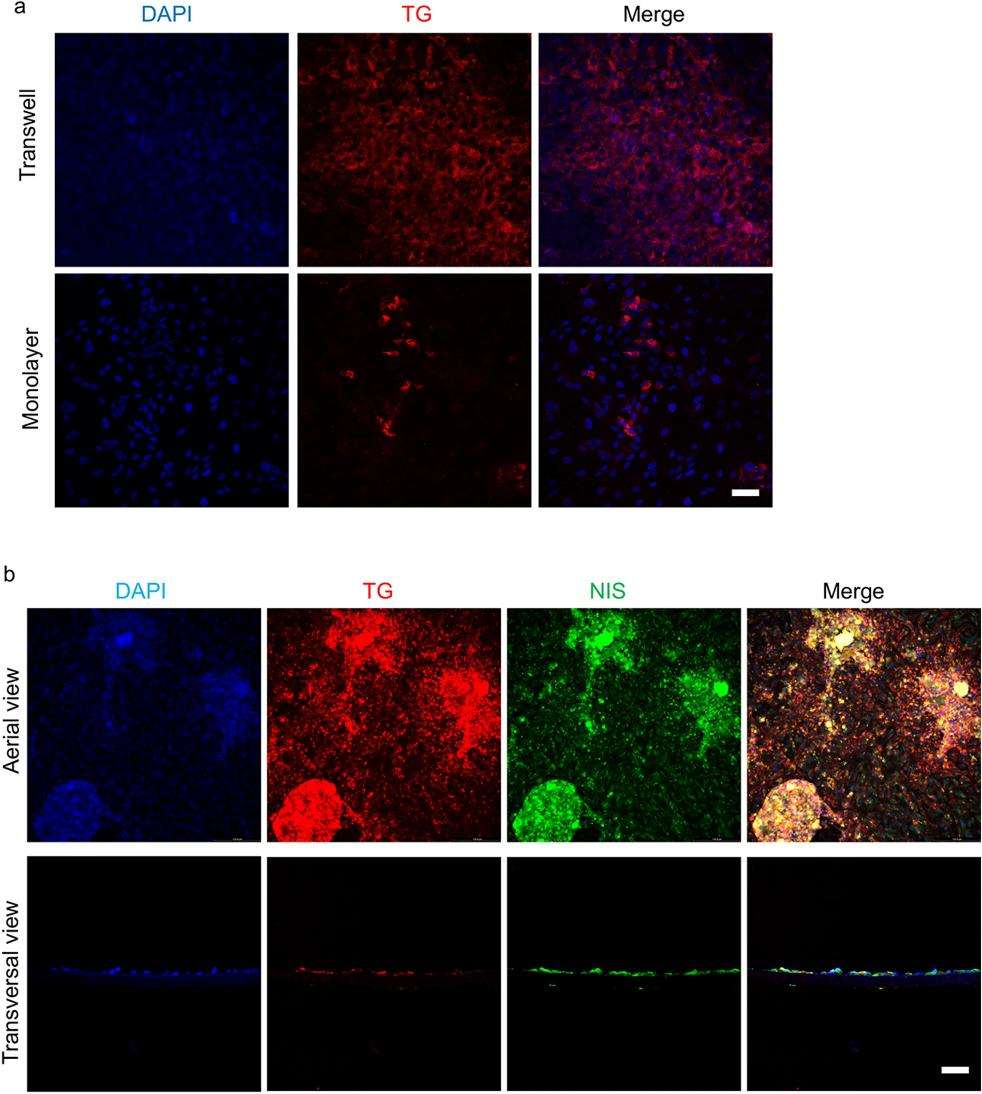 Fig. 3. The immunofluorescent staining of TG and NIS in primary human thyrocytes (Jiang B, Wang C, et al., 2023).
Fig. 3. The immunofluorescent staining of TG and NIS in primary human thyrocytes (Jiang B, Wang C, et al., 2023).
Ask a Question
Write your own review

