ONLINE INQUIRY

Human Testicular Endothelial Cells
Cat.No.: CSC-C9381W
Species: Human
Source: Testis
Morphology: Polygonal
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human testicular endothelial cells are derived from the normal blood vessels of human testicular tissue. Under a microscope, they typically exhibit a cobblestone or irregular shape. HTECs express specific markers such as CD31 and von Willebrand factor (vWF), which are used for their identification and purity assessment. During embryonic development, these cells migrate from the mesonephros and are involved in forming the vascular system of the testis during testicular cord formation. They are primarily located within the tunica vasculosa of the testis and not only construct the vascular system to maintain proper blood supply but may also play key roles in testicular physiological functions and germ cell development. Furthermore, studies indicate that testicular brain endothelial cells share similar properties with testicular vascular endothelial cells, aiding in the establishment of the blood-testis barrier (BTB), which is crucial for maintaining the stable environment of the testis and protecting germ cells from external damage.
This cell line holds significant application value in studying male reproductive physiology, such as the regulation of the spermatogenic microenvironment and the vascular mechanisms of testosterone secretion. It also serves as an important model for researching testicular diseases. In testicular tumor studies, it can be used to explore tumor angiogenesis mechanisms and evaluate the effectiveness of anti-angiogenic therapy strategies. In inflammatory diseases like orchitis, it helps in understanding changes in endothelial cells and the mechanisms of immune cell migration during inflammation.
Characterizations and Proteomic Analysis of TECs-Exos
TECs play an integral role in maintaining spermatogonial stem cells by producing specific factors vital for spermatogenesis. Exosomes facilitate cell communication, thus influencing physiological processes including sperm formation. However, the involvement of TECs in spermatogenesis has not been extensively studied.
Song et al. leveraged ultracentrifugation to isolate HTEC-Exos, followed by characterization through nanoparticle tracking analysis, TEM, and western blotting. The exosomes were round or elliptical, most of them between 70-200 nm in diameter (Fig. 1a and 1b). Exosomal markers TSG101, CD63 and CD9 were found by Western blotting but not the endoplasmic reticulum marker calnexin (Fig. 1c). HTEC-Exos had 945 proteins and 5470 peptides in LC-MS. The proteins were identified with more than half (562 of 945, 59.5%) containing 2 different peptides (Fig. 2a), which made the identification confidence high. KEGG ranking indicated the top 20 signaling pathways, where the number of "Ribosome", "Focal adhesion" and "ECM-receptor interaction" pathways are very rich (Fig. 2b). GO analysis oriented around molecular activity, cellular component, and biology. Proteins were high in "cell-cell adhesion", "signal transduction", and "translational initiation" (Fig. 2c), but also in "extracellular exosome", "cytosol" and "cytoplasm" (Fig. 2d). Molecular activities were "binding proteins", "binding poly(A) RNA" and "binding ATP" (Fig. 2e).
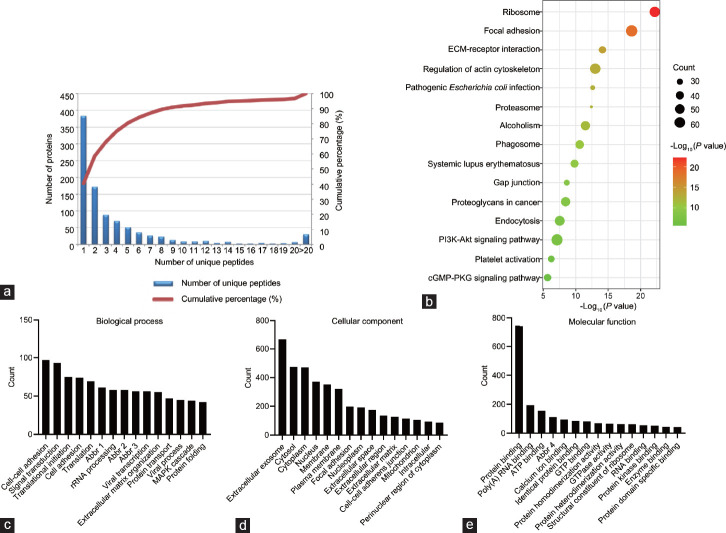 Fig. 1. Proteomic analyses of the exosomes from testicular endothelial cells (Song WP, Gu SJ, et al., 2022).
Fig. 1. Proteomic analyses of the exosomes from testicular endothelial cells (Song WP, Gu SJ, et al., 2022).
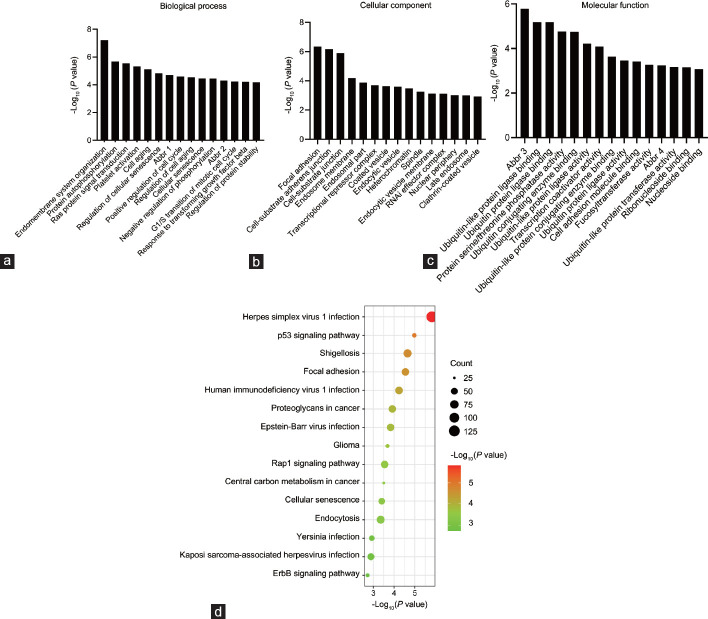 Fig. 2. miRNA profiling of the exosomes from testicular endothelial cells (Song WP, Gu SJ, et al., 2022).
Fig. 2. miRNA profiling of the exosomes from testicular endothelial cells (Song WP, Gu SJ, et al., 2022).
Human-Mouse Comparisons in Intra-Niche and Niche-Germline Interaction
Human spermatogenesis involves SSC differentiation into mature sperm, regulated by the testis niche. While progress in understanding these processes has been made in mice, less is known about human SSC regulation. Using single-cell RNA sequencing, Guo et al. analyzed ~6500 unselected testicular cells from young adults via the 10× Genomics Chromium platform, providing a transcriptional atlas.
In human testicular endothelial cells (Cluster 10, marked by VWF and PECAM1), NOTCH signaling components (NOTCH4, JAG1, HES1, and MAML1) were up-regulated (Fig. 2b). Hedgehog pathway receptors (PTCH1, PTCH2) and downstream elements (GLI and IGFBP6) were highly expressed in human adult myoid (Cluster 11, marked by MYH11 and ACTA2) and Leydig cells, indicating ongoing Hedgehog and NOTCH signaling in adult human testes (Fig. 3c). Sertoli cells (Cluster 12, marked by SOX9 and AMH) express ITGA6, present in seminiferous tubule membranes (Fig. 3d). Sertoli cells also express WFDC2 and PRND, related to sperm maturation and interaction (Fig. 3d). Leydig cells (Cluster 13, marked by DLK1 and IGF1) expressed IGF binding proteins (IGFBP5, IGFBP3), INHBA, VIT, and testosterone biosynthesis genes STAR and HSD17B3. Responsive genes SHBG and SRD5A2 appeared in maturing sperm (Fig. 3f). Leydig and myoid cells expressed RA synthesis enzymes ALDH1A1 and ALDH1A3; STRA8 was present during spermatogonia to spermatocytes transition (Fig. 3f). WNT2B was in myoid cells, its receptors in primary spermatocytes, suggesting a meiosis role (Fig. 3f). PDGFB in endothelial cells, with receptors in Leydig and myoid cells, suggests endothelial influence via niche cell interaction. These data reveal similarities and differences in germline-niche interactions between humans and mice, paving the way for detailed studies.
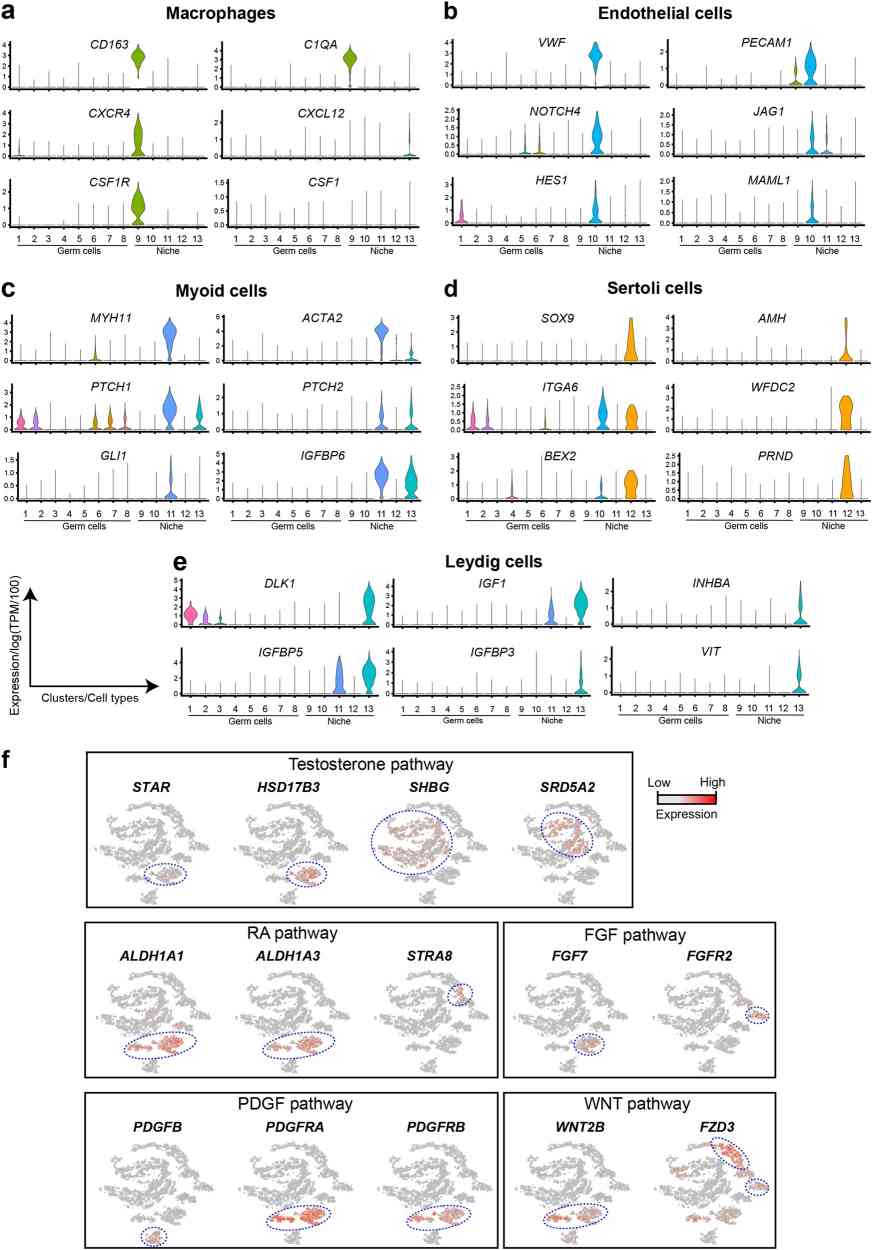 Fig. 3. Expression patterns of representative genes marking niche cells, and Niche-Germline interactions (Guo J, Groe E J, et al., 2018).
Fig. 3. Expression patterns of representative genes marking niche cells, and Niche-Germline interactions (Guo J, Groe E J, et al., 2018).
There are no set culture conditions for culturing a particular cell type. It is likely that cells cultured in MEM will grow just as readily in DMEM or M199. In summary, MEM is preferred for adherent cell cultures, and RPMI-1640 for suspension cell cultures is a good place to start.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Good growth
Cells grow well without any abnormalities in the medium.
25 July 2021
Ease of use
After sales services
Value for money
Write your own review