ONLINE INQUIRY
Human Pulmonary Artery Endothelial Cells
Cat.No.: CSC-C8602W
Species: Human
Source: Lung; Artery
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Pulmonary Artery Endothelial Cells (HPAECs) are human pulmonary arterial endothelial cells derived from human pulmonary arteries, which are extensively used to investigate pulmonary disease, especially the pathology of PAH. The pulmonary artery is an important blood vessel, which carries blood sent from the right ventricle to the lungs for gas exchange. It has three layers of wall, intima, media and adventitia, with endothelial cells in the innermost layer of the intima, closest to the blood supply. They can create and release chemicals that stimulate and disable the coagulation and fibrinolytic machinery that control blood clotting and dissolution. These cells can also make and secrete mediators of platelet adhesion and aggregation, and molecules that control cell proliferation and vascular wall tension. These roles make HPAEC relevant to the normal physiological and pathological function of the cardiovascular system. For instance, pulmonary arterial hypertension is a terminal pulmonary vascular disease caused by pulmonary artery endothelial cell dysfunction. With HPAEC, scientists can also deeply probe processes of angiogenesis, vascular remodeling and permeability regulation, and their association with cardiovascular disease.
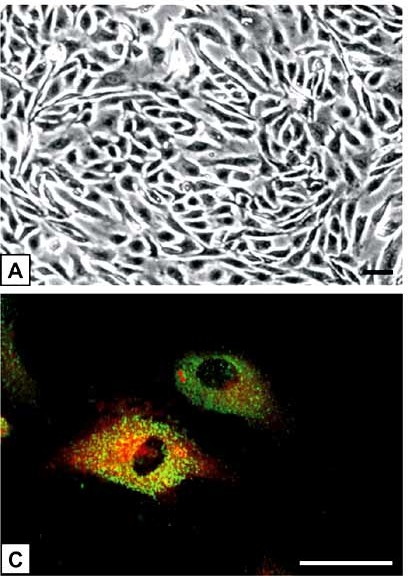 Fig. 1. (A) The polygonal-shaped HPAEC grow in loose arrangements and exhibit the classical cobblestone appearance at confluence. (C) Double-fluorescence staining showed a bright homogenous fluorescence of HPAEC with UEA-I lectin (red), in contrast to granular immunofluorescence with the von Willebrand factor antibody (green) (Hiden U, Lang I, et al., 2009).
Fig. 1. (A) The polygonal-shaped HPAEC grow in loose arrangements and exhibit the classical cobblestone appearance at confluence. (C) Double-fluorescence staining showed a bright homogenous fluorescence of HPAEC with UEA-I lectin (red), in contrast to granular immunofluorescence with the von Willebrand factor antibody (green) (Hiden U, Lang I, et al., 2009).
PAH HPAECs Had Higher Glucose Uptake with Reduced Lactate Secretion
Pulmonary arterial hypertension (PAH) is an incurable, progressive condition predominantly impacting women, with a global prevalence of 1%. Endothelial cell dysfunction, characterized by decreased production of the vasodilator nitric oxide (NO) and increased proliferation, is crucial in PAH pathology.
Basehore et al. therefore hypothesized that elevated endothelial glycolytic activity in PAH endothelial cells would reduce nitric oxide production by increasing eNOS O-GlcNAcylation. They found that disturbed flow increases glycolytic activity in endothelial cells, reducing nitric oxide through increased eNOS O-GlcNAcylation. To see if PAH patients have similar effects, they examined HPAECs from non-PAH and PAH patients. YSI Bioanalyzer results indicated PAH HPAECs showed over three times more glucose uptake than non-PAH cells (Fig. 1A), with lower lactate secretion, though not significant (Fig. 1B). The lactate/glucose ratio was significantly lower in PAH HPAECs (Fig. 1C) excluding two outliers. This indicates PAH HPAECs consume more glucose but produce less lactate. Using 13C6 glucose mass spectrometry, they found an increase in total glycolytic metabolite pools in PAH HPAECs compared to non-PAH, though not statistically significant due to high variability (Fig. 2A). The labeled fraction of glycolytic metabolites did not differ (Fig. 2B), suggesting increased glucose uptake raises glycolytic intermediates, but relative glycolysis flux is unchanged.
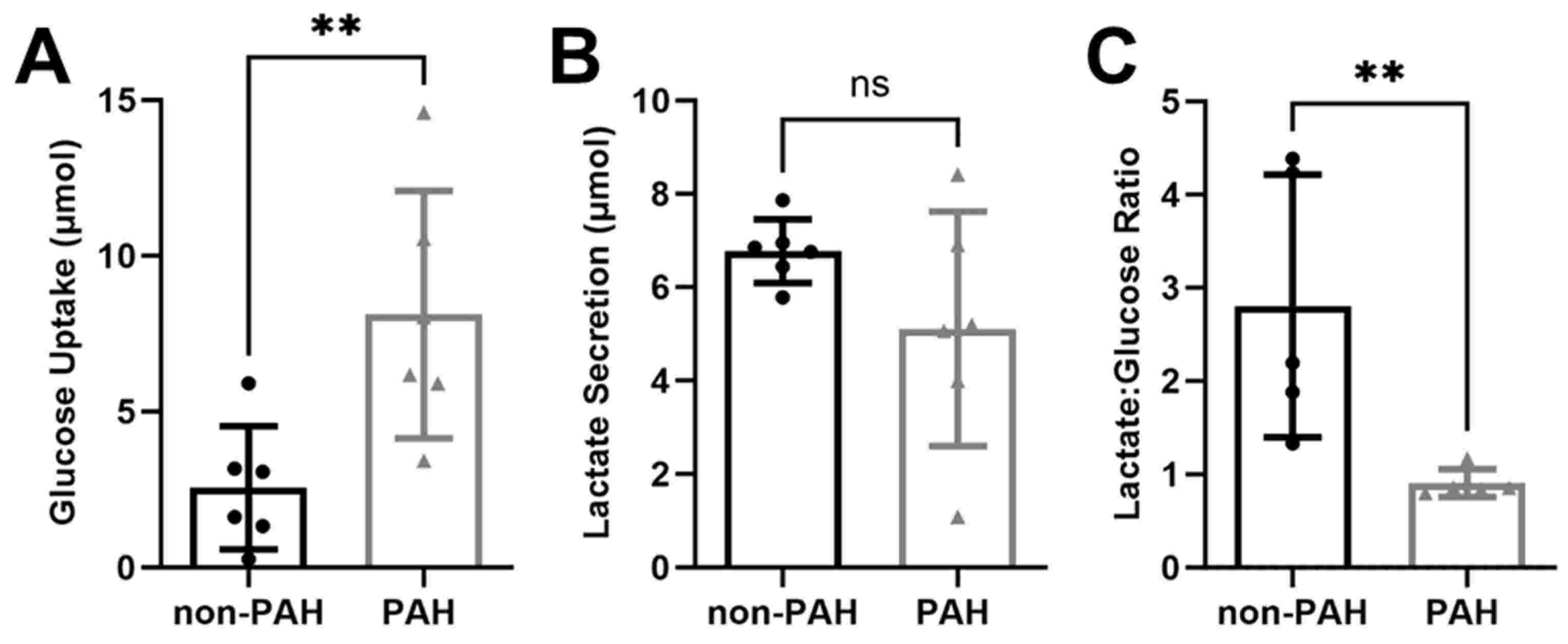 Fig. 1. PAH HPAECs consumed more glucose and produced less lactate compared to non-PAH HPAECs (Basehore S E, Clyne A M, et al., 2023).
Fig. 1. PAH HPAECs consumed more glucose and produced less lactate compared to non-PAH HPAECs (Basehore S E, Clyne A M, et al., 2023).
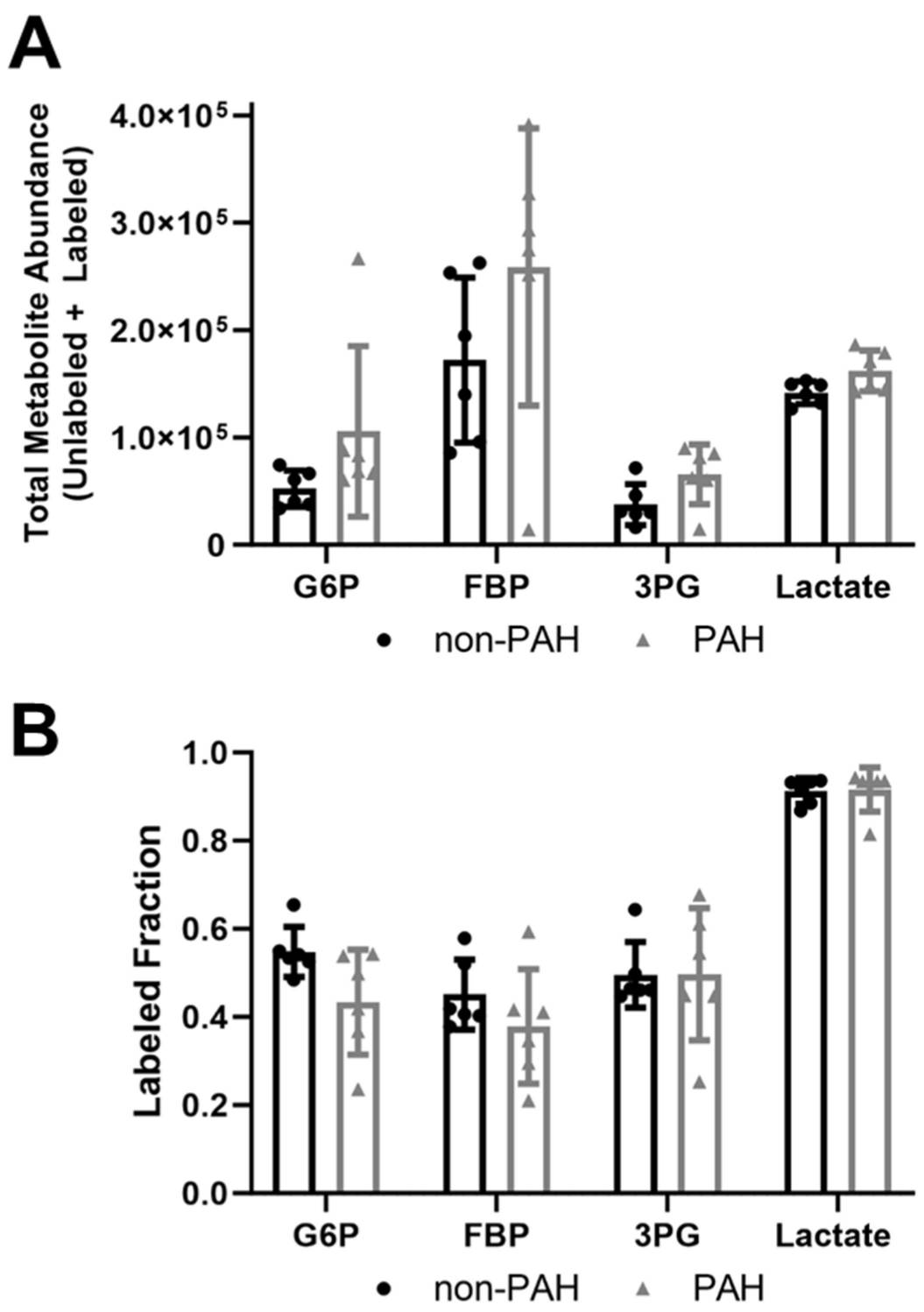 Fig. 2. Total glycolytic metabolite abundances were higher in PAH HPAECs than non-PAH HPAECs but the labeled fraction did not change (Basehore S E, Clyne A M, et al., 2023).
Fig. 2. Total glycolytic metabolite abundances were higher in PAH HPAECs than non-PAH HPAECs but the labeled fraction did not change (Basehore S E, Clyne A M, et al., 2023).
Knockdown of circHIPK3 Inhibited PDGF-Induced Proliferation, Migration and Angiogenesis of hPAECs
Acute pulmonary arterial hypertension (PAH) is characterized by elevated blood pressure and vascular remodelling. Endothelial cell dysfunction drives the development of PAH's. circRNA controls the function of vascular endothelial cells. Therefore, circRNA could play a key role in the expansion, migration and growth of human pulmonary artery endothelial cells (hPAECs) during pulmonary arterial hypertension. So, Hong's team investigated the circHIPK3's influence on PAH by using a model with PDGF-treated hPAECs.
In order to investigate circHIPK3's contribution to PDGF-induced hPAECs, three siRNAs were produced to down-regulate its expression. qRT-PCR results showed that siRNA-2 performed best (Fig. 3a), so it was adopted for additional tests. Transfection with circHIPK3 siRNA dramatically reduced circHIPK3 expression in PDGF-treated hPAECs (Fig. 3b). In CCK-8 assays, circHIPK3 knockdown inhibited PDGF-induced hPAEC proliferation (Fig. 3c). In hPAECs, transwell experiments confirmed that knockdown of circHIPK3 reduced the proliferation of cells driven by PDGF (Fig. 3d). Tube formation assays showed diminished capillary-like structure generation (Fig. 3e). Further, qRT-PCR and western blot experiments showed that circHIPK3 knockdown inhibited PDGF's effect on bFGF and VEGF expression at mRNA and protein levels (Fig. 3f and g). These findings suggest that circHIPK3 knockdown blocks PDGF-dependent proliferation, migration and angiogenesis in hPAECs.
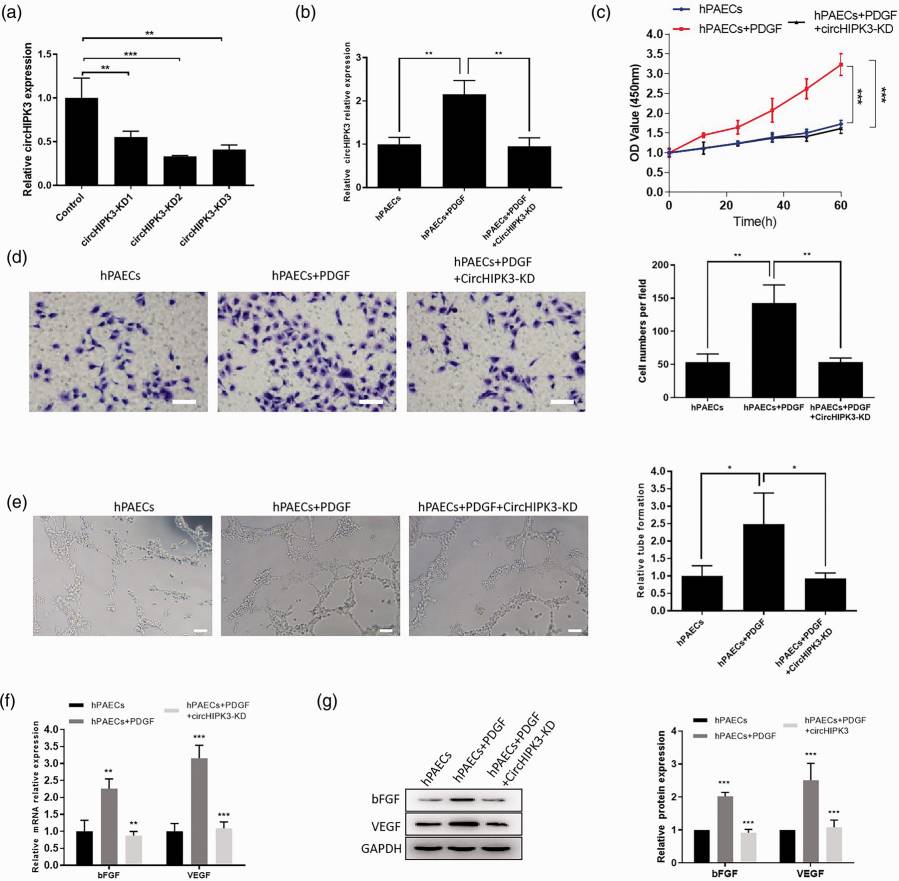 Fig. 3. CircHIPK3 regulated the function of PDGF-treated hPAECs (Hong L, Ma X, et al., 2021).
Fig. 3. CircHIPK3 regulated the function of PDGF-treated hPAECs (Hong L, Ma X, et al., 2021).
Ask a Question
Write your own review

