ONLINE INQUIRY

Human Placental Vascular Endothelial Cells
Cat.No.: CSC-C9338W
Species: Human
Source: Placenta
Morphology: Polygonal
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human placental vascular endothelial cells (HPVECs) are a primary cell line used to study placental vascular development and function. The placenta, the major channel of material transfer between the fetus and mother, is made up of the amnion, chorionic villi and decidua. HPVECs are typically isolated from the chorionic villi or amnion and show the appearance of mature arteries and juvenile veins, as well as potential differentiation for adipogenesis and osteogenesis. The vasculature in the chorionic villi emerges from umbilical veins and spreads widely through the placenta. This network offers crucial routes for gas exchange, nutrients, and metabolic waste elimination between the fetus and mother.
HPVECs are oblong-shaped and distributed in ragged layers. They show a rapid growth rate when exposed to vascular endothelial growth factor (VEGF). They are closely associated with several maternal conditions affecting pregnancy, including placental insufficiency, intrauterine growth restriction, and preeclampsia, which makes them an excellent in vitro tool for understanding the cause and treatment of these disorders. As regenerative medicine moves forward, HPVECs could also play an important role in vessel regeneration and tissue repair.
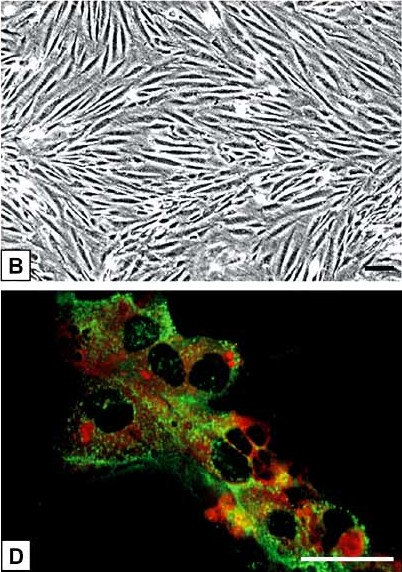 Fig. 1. (B)The spindle-shaped HPVEC grow closely apposed to each other and form monolayers with swirling morphology. (D) Double-fluorescence staining showed a bright homogenous fluorescence of HPVEC with UEA-I lectin (red), in contrast to granular immunofluorescence with the von Willebrand factor antibody (green) (Hiden U, Lang I, et al., 2009).
Fig. 1. (B)The spindle-shaped HPVEC grow closely apposed to each other and form monolayers with swirling morphology. (D) Double-fluorescence staining showed a bright homogenous fluorescence of HPVEC with UEA-I lectin (red), in contrast to granular immunofluorescence with the von Willebrand factor antibody (green) (Hiden U, Lang I, et al., 2009).
GDF-15 Alleviates Hypoxia-Reoxygenation-Induced Damage to Human Placental Vascular Endothelial Cells by Regulating SIRT1
Pregnancy-induced hypertension (PIH) is a common condition that stems from the dysfunction of maternal endothelial cells, with the involvement of factors like SIRT1 and Growth differentiation factor 15 (GDF-15). GDF-15, part of the TGF-β superfamily, is crucial for cardiovascular protection and its low levels are associated with PIH. H/R-induction in human placental vascular endothelial cells (HPVECs) is a standard method for developing an in vitro PIH model.
Chen's team used HPVECs in vitro exposed to GDF-15 to study the mechanism of how GDF-15 can stave off H/R-mediated damage to HPVECs. The induction of H/R significantly reduced the proliferation of HPVECs (Fig. 1A). When H/R-induced HPVECs reacted with 1 ng/mL, 10 ng/mL and 100 ng/mL GDF-15, it was 100 ng/mL GDF-15 that significantly overcame the inhibited viability (Fig. 1B). Therefore, a dose of 100 ng/mL GDF-15 was used for the in vitro studies that followed. Sirtuin 1 (SIRT1) is a member of the sirtuin family that safeguards endothelial cells from senescence. H/R induction in HPVECs dramatically reduced SIRT1 mRNA and protein levels which were partially upregulated by GDF-15. Interestingly, GDF-15's regulatory function in turning back H/R-mediated downregulation of SIRT1 in HPVECs was inhibited by the AMPK inhibitor C (Fig. 2).
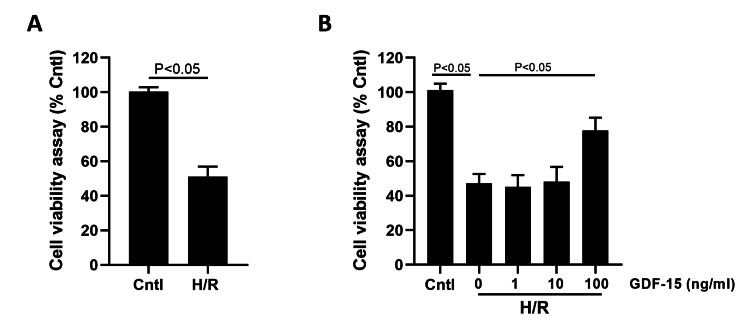 Fig. 1. GDF-15 increased the viability of H/R-suppressed HPVECs (Chen C and Shi J, 2024).
Fig. 1. GDF-15 increased the viability of H/R-suppressed HPVECs (Chen C and Shi J, 2024).
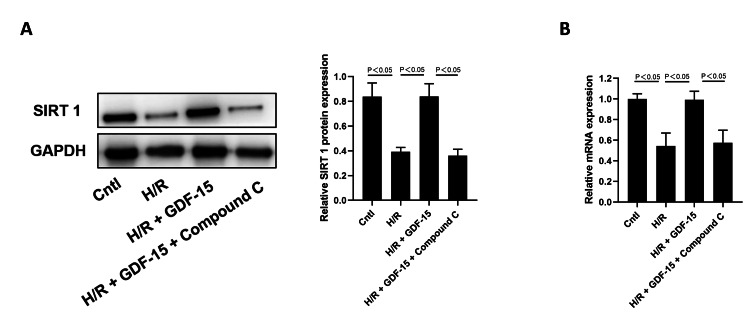 Fig. 2. GDF-15 reversed H/R-induced downregulation of SIRT1 in HPVECs (Chen C and Shi J, 2024).
Fig. 2. GDF-15 reversed H/R-induced downregulation of SIRT1 in HPVECs (Chen C and Shi J, 2024).
CTRP9 Overexpression Retards Cell Viability Loss and Apoptosis, and Alleviates Migration and Angiogenesis of HPVECs Mediated by H/R
Invasive maternal and fetal deaths have been reported from 6-10% of all pregnancies worldwide due to pregnancy-related hypertension (PIH). It is defined by diffuse endothelial degeneration and angiogenic diseases that cause fetal hypoxia. CTRP9 is a protein of the adiponectin family that has been shown to be protective of the cardiovascular system and to improve endothelial dysfunction in PIH.
Lin et al. used human placental vascular endothelial cells (HPVECs) treated with H/R to induce PIH in vitro. CTRP9 overexpression was analyzed for its impact on cell viability, apoptosis, migration, and angiogenesis through assays like flow cytometry and PCR. As shown in Fig. 3C-D, CTRP9 levels decreased with H/R induction but significantly increased after oe-CTRP9 transfection. The CCK-8 assay revealed reduced cell viability post-H/R, which was notably improved by CTRP9 overexpression (Fig. 3E). H/R increased apoptosis, partially inhibited by CTRP9 overexpression (Fig. 3F). Additionally, the decrease in Bcl-2 and increase in Bax and cleaved-caspase3 from H/R were partially reversed with CTRP9 overexpression (Fig. 3G), confirming CTRP9's anti-apoptotic role. HPVEC migration and tube formation reduced after H/R but improved with CTRP9 overexpression (Fig. 4A-B). Tube formation assays show angiogenic capability; thus, CTRP9 overexpression enhanced both migration and angiogenesis in H/R-exposed HPVECs.
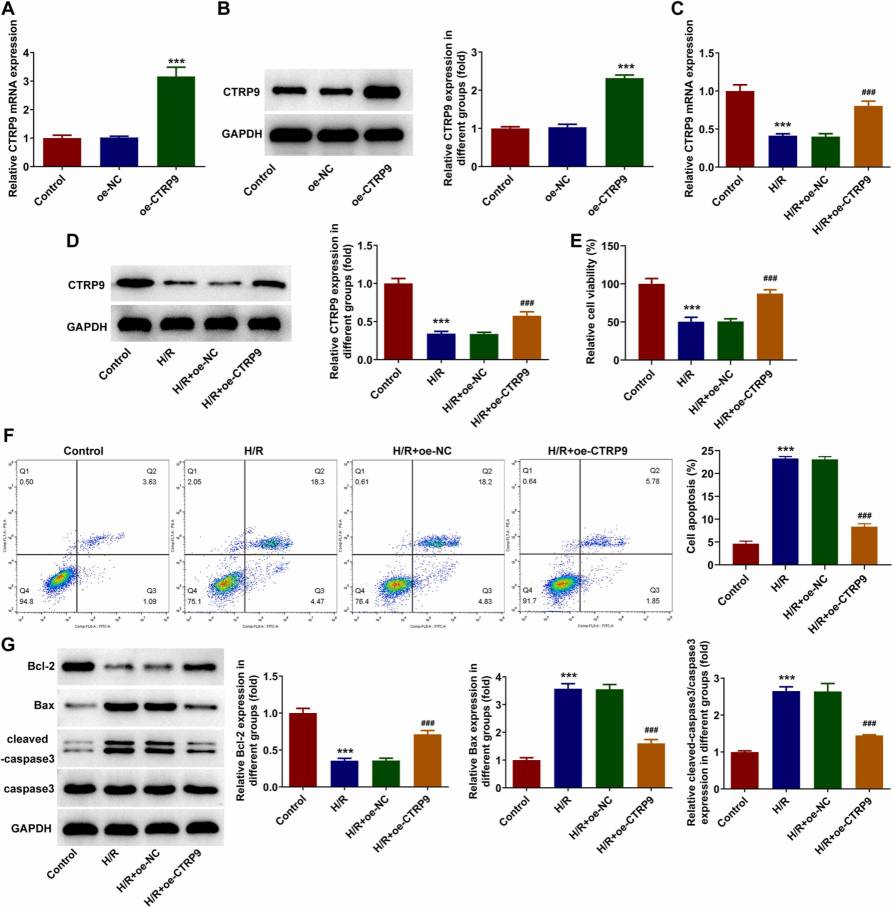 Fig. 3. CTRP9 overexpression retards H/R-mediated cell viability loss and apoptosis of HPVECs (Zhu L, Chen S, et al., 2023).
Fig. 3. CTRP9 overexpression retards H/R-mediated cell viability loss and apoptosis of HPVECs (Zhu L, Chen S, et al., 2023).
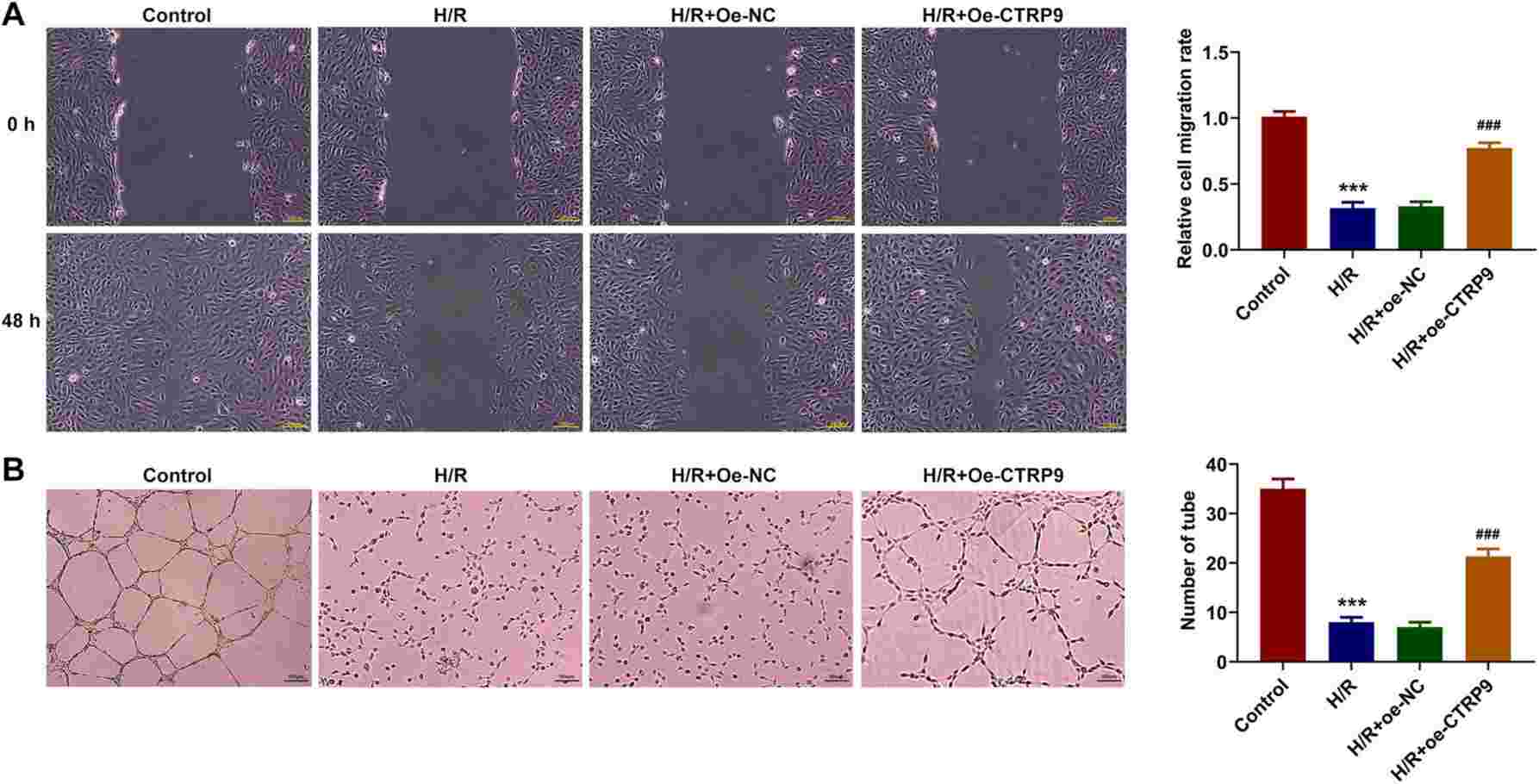 Fig. 4. CTRP9 overexpression alleviates H/R-mediated impaired migration and angiogenesis in HPVECs (Zhu L, Chen S, et al., 2023).
Fig. 4. CTRP9 overexpression alleviates H/R-mediated impaired migration and angiogenesis in HPVECs (Zhu L, Chen S, et al., 2023).
Cells are not shaken well after passaging into the incubator, or they are shaken well when they are put in but then the flasks are moved before the cells are attached to the wall, vibration caused by frequent opening and closing of the incubator or there is too little culture solution in the flasks, and the surface of the incubator shelves is not flat, which will lead to uneven growth of the cells.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Stable and cost-effective
Our laboratory has long used Creative Bioarray's cellular products, which are stable and cost-effective.
06 May 2021
Ease of use
After sales services
Value for money
Write your own review

