ONLINE INQUIRY

Human Mammary Fibroblasts
Cat.No.: CSC-C1500
Species: Human
Source: Breast
Morphology: Fibroblasts
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
HMF are isolated from human mammary tissue. HMF are cryopreserved at primary culture and delivered frozen. Each vial contains >5 x 10^5 cells in 1 ml volume. HMF are characterized by immunofluorescent method with antibodies to fibronectin, CD90 and vimentin. HMF are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HMF are guaranteed to further expand for 15 population doublings at the conditions provided by Creative Bioarray.
Never can primary cells be kept at -20 °C.
The source of human mammary fibroblasts is breast tissue which represents a complex organ consisting of glands known as mammary parenchyma and supportive fibrous tissue called stroma. Mammary stroma contains fibroblasts which perform essential functions in breast development as well as tissue repair and immune system regulation. These cells secrete extracellular matrix elements including collagen that offer structural support to epithelial cells and enable branching duct development.
Human mammary fibroblasts become essential elements in breast cancer research. Research demonstrates that human mammary fibroblasts support breast cancer cell invasion and metastasis through the secretion of tumor-promoting substances including IL-6 and MMP-3. The interaction between fibroblasts and cancer cells establishes a tumor microenvironment that boosts cancer growth while helping cancer cells evade the immune system. During tissue repair processes these cells construct synthetic tissues or mend existing damaged tissues. During breast reconstruction surgery fibroblasts help create new tissue by producing collagen and other components of the extracellular matrix.
 Fig. 1. Human Mammary Fibroblasts (Archer M, Dasari P, et al., 2022).
Fig. 1. Human Mammary Fibroblasts (Archer M, Dasari P, et al., 2022).
Global Histone Deacetylation and TGF-β-Smad2/3 Signaling Activation are Induced in Gln-starved HMFs in a Class I HDAC-dependent Fashion
The TME shapes carcinoma-associated fibroblasts (CAFs) function through glutamine scarcity which influences tumor growth by interfering with metabolic pathways such as transforming growth factor-β (TGF-β) and mechanistic/mammalian target of rapamycin complex 1 (mTORC1) signaling. Mezawa et al. investigated how glutamine deprivation leads to TGF-β signaling activation through class I HDAC activity and mTORC1 suppression in human mammary fibroblasts (HMFs) and ultimately drives their transformation into myCAFs and cancer progression.
They studied the effects of glutamine deprivation on TGF-β signaling and histone modification by culturing HMFs under glutamine-starved conditions in vitro. The total histone acetylation levels of H3 and H4 diminished by 65.7% and 53.8% respectively in glutamine-starved HMFs compared to their glutamine-supplemented counterparts (Fig. 1A). The histone deacetylation process reflects myCAF features observed in breast cancer which indicates that a lack of glutamine triggers TGF-β signaling activation which serves as a myCAF signature. The ratio of phosphorylated Smad2 to Smad2/3 rose 3.6 times which signifies enhanced TGF-β signaling (Fig. 1B). The mRNA levels of TGFB1, TGFB2, SERPIN1, and SMAD7 TGF-β genes increased except for ACTA2 as shown in Figure 1C. 1C). HDAC inhibitors such as trichostatin A (TSA) caused dose-dependent increases in acH3/H3 and acH4/H4 levels while decreasing pSmad2/Smad2/3 ratios (Fig. 1D). 1D). SAHA and entinostat which are class I HDAC inhibitors demonstrated effectiveness in reducing pSmad2/Smad2/3 elevation under glutamine-deficient conditions (Fig. 1E). 1E). The deficiency of glutamine leads to histone deacetylation and TGF-β signaling through class I HDAC activation.
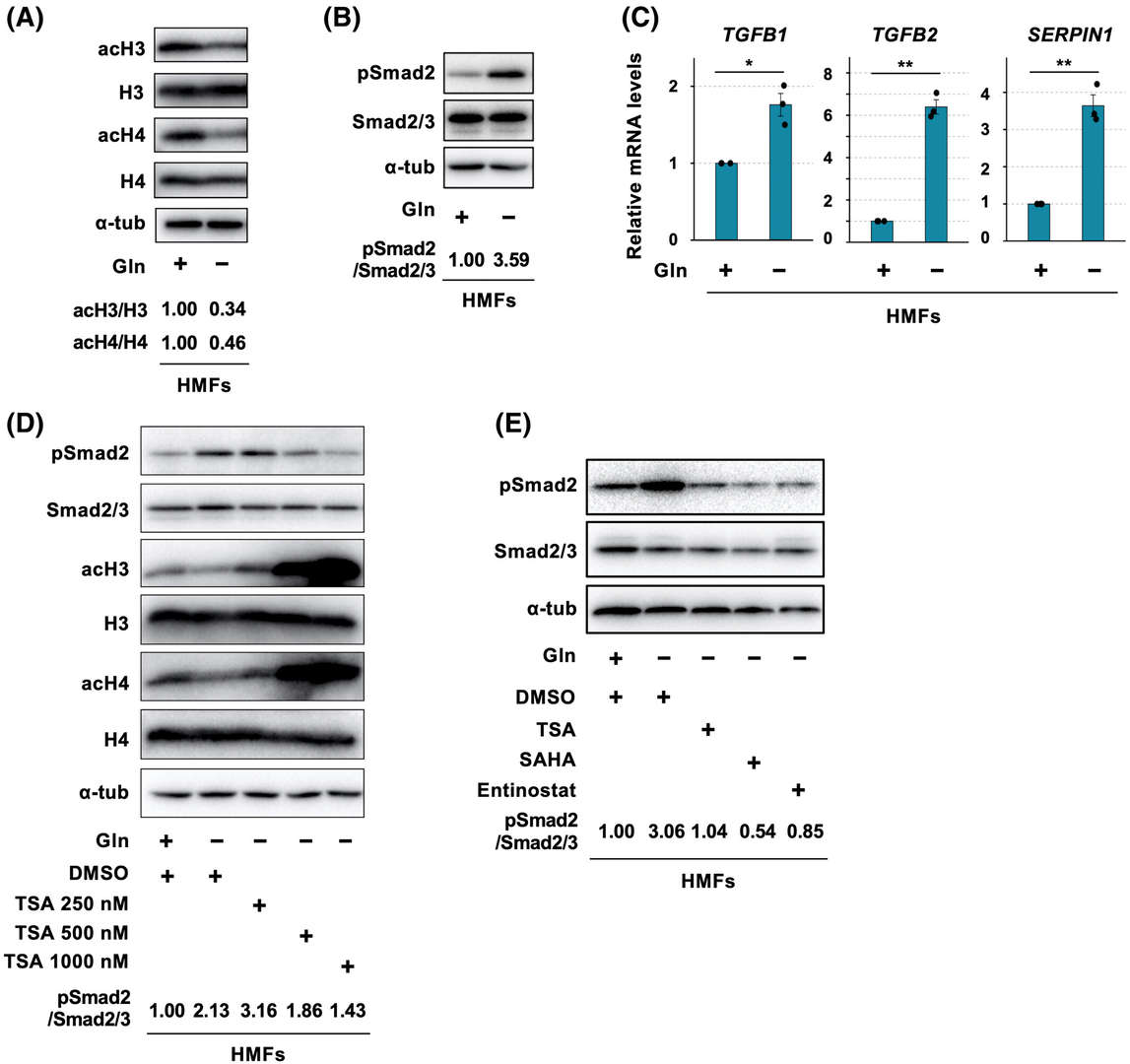 Fig. 1. Glutamine (Gln) starvation induces global histone deacetylation and transforming growth factor-β (TGF-β)-Smad2/3 signaling activation in human mammary fibroblasts (HMFs) in a class I histone deacetylase-dependent fashion (Mezawa Y, Wang T, et al., 2023).
Fig. 1. Glutamine (Gln) starvation induces global histone deacetylation and transforming growth factor-β (TGF-β)-Smad2/3 signaling activation in human mammary fibroblasts (HMFs) in a class I histone deacetylase-dependent fashion (Mezawa Y, Wang T, et al., 2023).
Morphologic Changes and Alterations in the Expression of CAF Markers after the TNC Treatment
Tenascin-C (TNC), an extracellular matrix glycoprotein, is highly expressed in breast cancer by both cancer and stromal cells. TNC expression is linked to worse outcomes in breast cancer, influencing tumor progression by promoting cancer cell proliferation, migration, and invasion.
Katoh et al. investigates how TNC affects the transformation of human mammary fibroblasts (HMFs) into cancer-associated fibroblasts (CAFs). After 4 days of TNC (10 μg/mL) treatment, HMFs mainly exhibited a spindle shape on plastic and formed more clusters on glass, similar to smooth muscle cells (Fig. 2A). TNC significantly increased α-SMA and calponin expression, indicating differentiation into myofibroblasts, but not high-molecular-weight caldesmon (Fig. 2B and C). The upregulation of platelet-derived growth factor receptor-β suggested potential smooth muscle cell differentiation. There were no significant changes in fibroblast-activation protein α, neural/glial antigen 2, prolyl-4-hydoroxylase, podoplanin, or S100A4 expressions. TNC likely induces differentiation into myofibroblasts rather than other CAF subsets. Immunofluorescence showed that treated HMFs developed prominent stress fibers with positive α-SMA/calponin staining, while untreated cells had scant fibers (Fig. 2D).
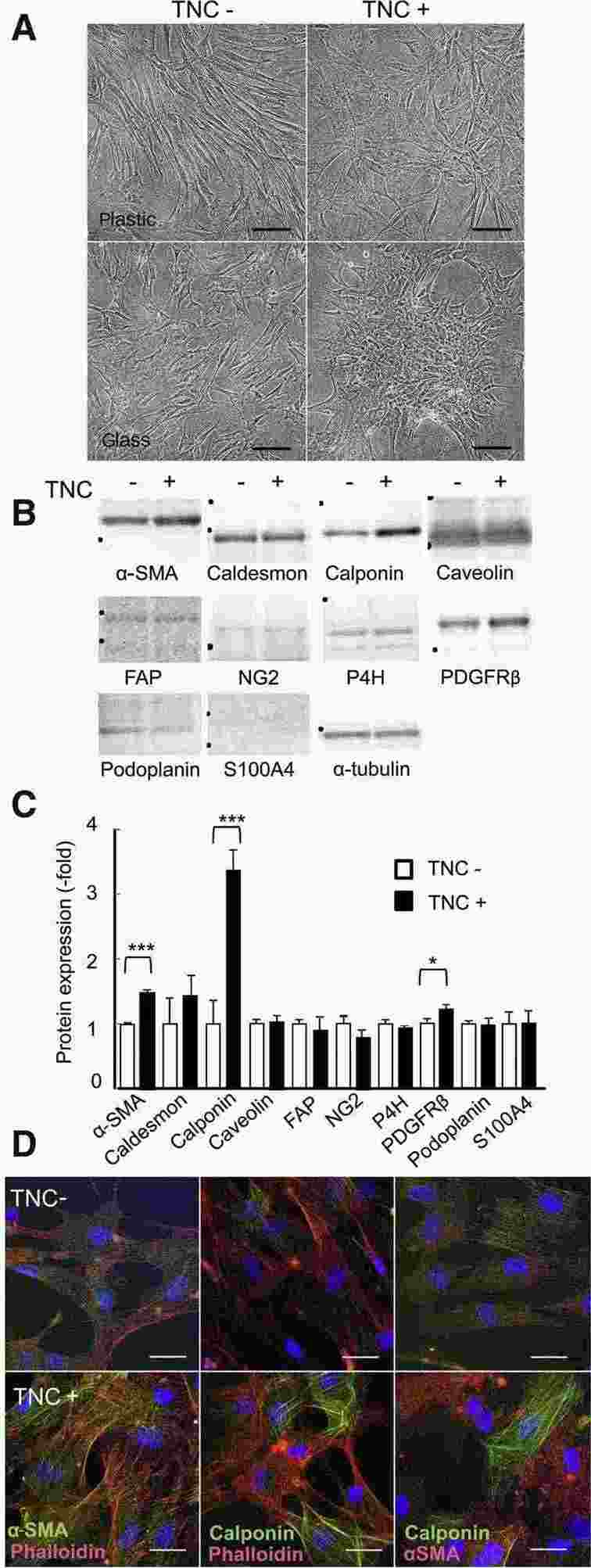 Fig. 2. Morphologic changes and the altered expression of cancer-associated fibroblast (CAF) markers in human mammary fibroblasts (HMFs) after the tenascin-C (TNC) treatment (Katoh D, Kozuka Y, et al., 2020).
Fig. 2. Morphologic changes and the altered expression of cancer-associated fibroblast (CAF) markers in human mammary fibroblasts (HMFs) after the tenascin-C (TNC) treatment (Katoh D, Kozuka Y, et al., 2020).
Ask a Question
Write your own review


