ONLINE INQUIRY

Human Internal Thoracic Artery Endothelial Cells
Cat.No.: CSC-C9332W
Species: Human
Source: Artery
Morphology: Polygonal
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human internal thoracic artery endothelial cells (HITAECs) are a line of endothelial cells that originate from the internal thoracic artery. This artery branches from the left side of the anterior part of the subclavian artery, where the vertebral artery starts, and enters the chest cavity behind the first to seventh costal cartilages and 1.5 cm from the lateral edge of the sternum. It is the primary vessel for coronary artery bypass grafting (CABG). The HITAECs produce markers such as vascular endothelial growth factor receptor (vWF/Factor VIII), CD31, and PECAM-1. As important regulators of blood vessel homeostasis, HITAECs secrete substances like NO to control blood vessel tone and reduce platelet accumulation. They are also involved in angiogenesis, inflammation and post-vascular damage repair.
Thus, HITAECs are widely used in studies to understand the functions of the vascular endothelium, including angiogenesis, inflammation and interactions with immune cells. For example, they have shown that these cells are resistant to endothelial disease in the internal thoracic arteries transplanted in patients with diabetes. Furthermore, the internal thoracic artery is more resistant to atherosclerosis than the greater saphenous vein, making HITAECs invaluable for understanding protective vascular processes.
MMC-induced Genotoxic Stress in HCAEC and HITAEC
Mitomycin C (MMC), an alkylating chemotherapy drug, causes DNA crosslinking, leading to genotoxic stress, endothelial dysfunction, and potentially atherosclerosis. Endogenous and exogenous agents, including MMC, can induce such DNA damage, which triggers inflammation and promotes atherosclerosis development via endothelial dysfunction.
Sinitsk et al. explored the effects of MMC-induced DNA damage on gene expression in human coronary artery endothelial cells (HCAEC) and human internal thoracic endothelial cells (HITAEC) by exposed to 500 ng/mL MMC in vitro. After 6 hours of MMC treatment and 24 hours in MMC-free media, the CBMN assay results for HCAEC and HITAEC are shown in Fig. 1. MMC-treated cells exhibited significant increases in genotoxic stress markers: (i) binucleated (BN) cells with micronuclei (MNi) from chromosome fragments or missegregated chromosomes, (ii) nucleoplasmic bridges (NPBs) from dicentric chromosome misrepair or telomere fusion, and (iii) nuclear buds (NBUDs) indicating DNA and protein complex repair (Fig. 2). MMC led to a 3-fold and 2-fold rise in BN cells with MN and NBUDs in HCAEC and HITAEC, respectively, and a 4-fold and 3-fold increase in BN cells with NPBs. Baseline cytogenetic markers varied between cell types, with HITAEC showing higher initial levels, notably in BN cells with NPBs, similar to MMC-treated HCAEC levels (Fig. 1). Despite these differences, the fold-change in cytogenetic markers due to MMC was similar for both endothelial cell types. These results confirm that alkylating mutagen MMC inflicts cytogenetic damage in primary human endothelial cells.
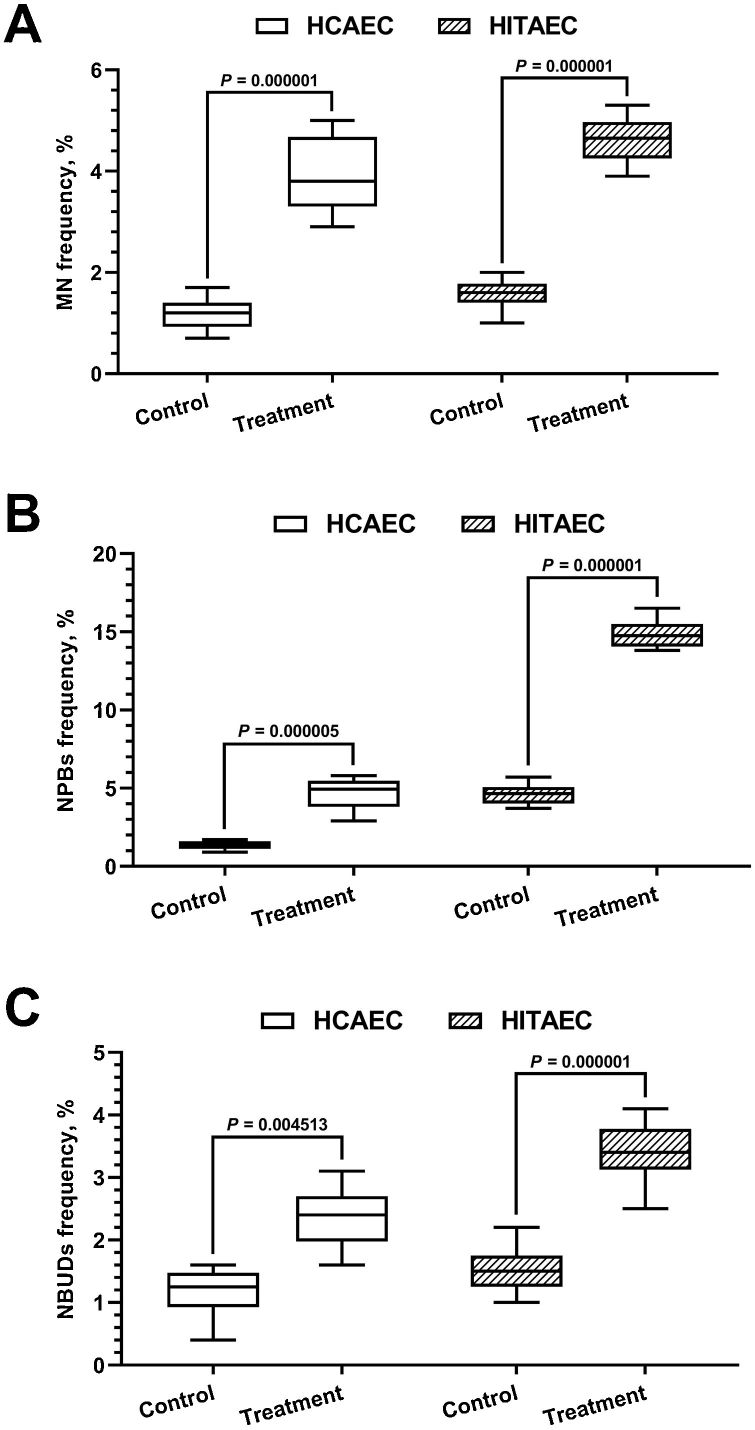 Fig. 1. Frequency of BN cells with MN (A), NPBs (B) and NBUDs (C) in HCAEC and HITAEC in response to 500 ng/mL MMC (Sinitsky M Y, Kutikhin A G, et al., 2020).
Fig. 1. Frequency of BN cells with MN (A), NPBs (B) and NBUDs (C) in HCAEC and HITAEC in response to 500 ng/mL MMC (Sinitsky M Y, Kutikhin A G, et al., 2020).
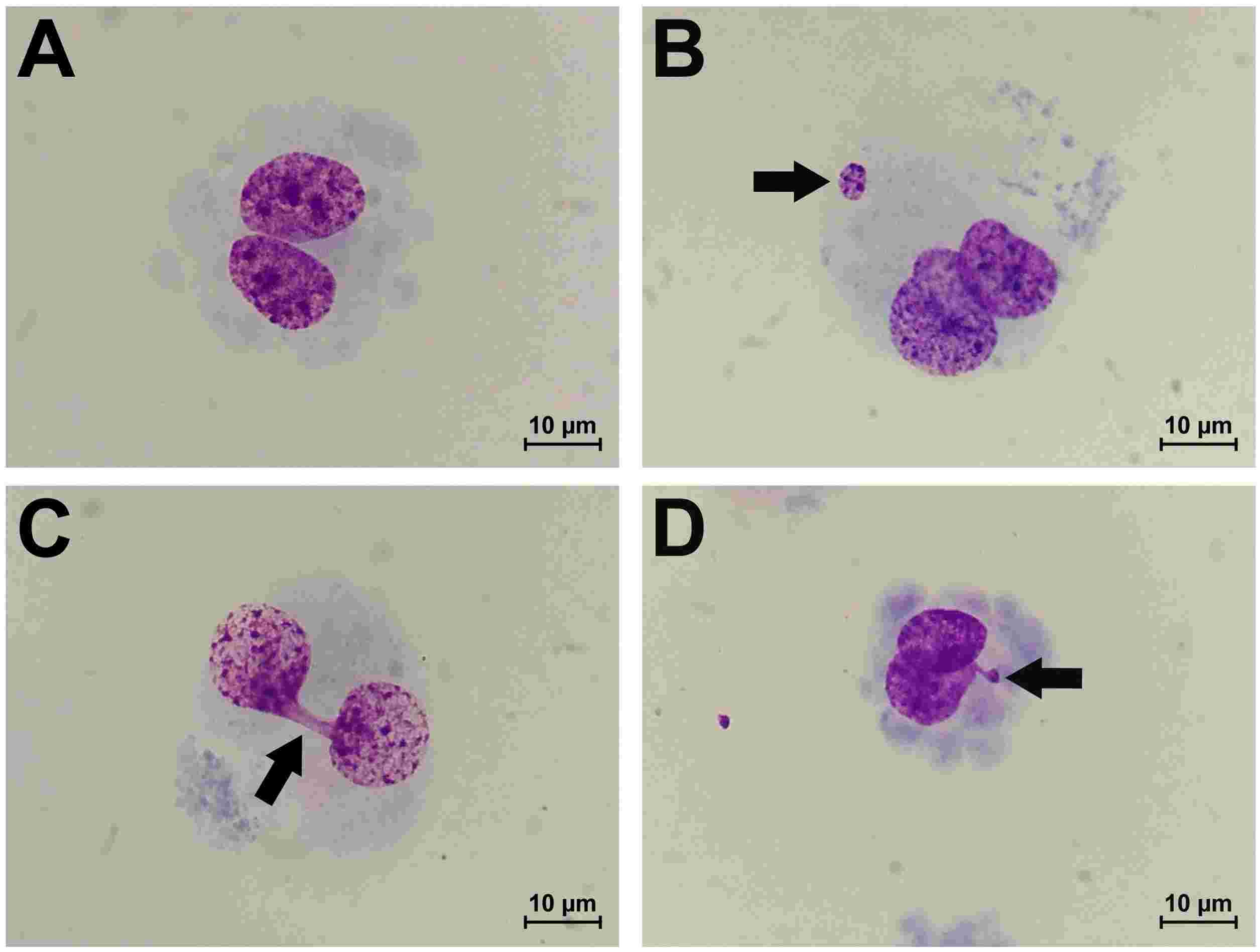 Fig. 2. Morphology of undamaged BN endothelial cell (A), BN cell with MNi (B), BN cell with NPB (C) and BN cell with NBUD (D) at ×1000 magnification (Sinitsky M Y, Kutikhin A G, et al., 2020).
Fig. 2. Morphology of undamaged BN endothelial cell (A), BN cell with MNi (B), BN cell with NPB (C) and BN cell with NBUD (D) at ×1000 magnification (Sinitsky M Y, Kutikhin A G, et al., 2020).
Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions
Coronary artery bypass grafting (CABG) is a common surgical intervention for coronary artery disease. Despite the superior long-term patency of internal thoracic artery (ITA) grafts over saphenous vein (SV) conduits due to better resistance to atherosclerosis and other complications, SVs remain commonly used. Shishkova's team explored the underlying reasons for ITA grafts' superior performance by investigating the synergistic paracrine interactions between coronary artery endothelial cells (HCAECs) and internal thoracic artery endothelial cells (HITAECs).
To examine if HITAECs provide beneficial paracrine effects to HCAECs unlike HSaVECs, and to identify distinct patterns in their interactions, they used a co-culture model with HCAECs at the dish bottom and conduit ECs (HITAECs or HSaVECs) on a translucent polycarbonate insert. RT-qPCR revealed reduced pro-inflammatory and EndoMT transcripts in HCAECs with HITAECs compared to HSaVECs (Fig. 3A). Specifically, IL6 and CXCL8 were downregulated after 24 and 48 hours, while genes VCAM1, ICAM1, SELE, SNAI1, and SNAI2 showed varied expression (Fig. 3A). Western blotting confirmed eNOS increase and consistent Snail/Slug reduction in HCAECs with HITAECs post 48 hours (Fig. 3B and C). Despite an unexpected VCAM1 rise, it remained lower than in monocultures. Upregulation of arterial specification transcription factor HES1 suggested HITAECs aid arterial differentiation in HCAECs, as opposed to HSaVECs which disrupt it (Fig. 3B and C). Both CD31 and VE-cadherin levels stayed unchanged, indicating endothelial identity changes occur beyond observed time frames.
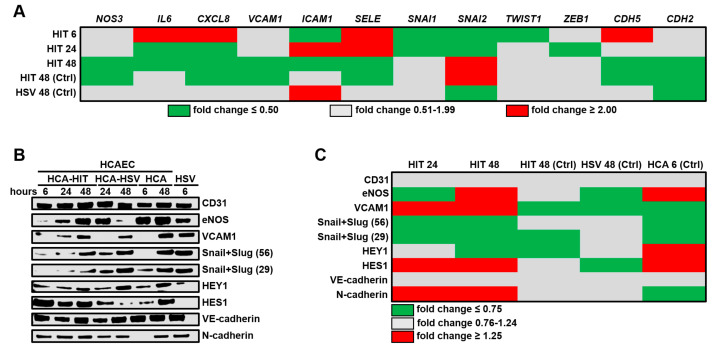 Fig. 3. Profiling of key endothelial molecules in human coronary artery endothelial cells (HCAECs) co-cultured with either human internal thoracic artery endothelial cells (HITAECs) (HIT) or human saphenous vein endothelial cells (HSaVECs) (HSVs) for 6, 24, or 48 h (Shishkova D, Markova V, et al., 2020).
Fig. 3. Profiling of key endothelial molecules in human coronary artery endothelial cells (HCAECs) co-cultured with either human internal thoracic artery endothelial cells (HITAECs) (HIT) or human saphenous vein endothelial cells (HSaVECs) (HSVs) for 6, 24, or 48 h (Shishkova D, Markova V, et al., 2020).
When cells are to be recovered during cell passaging or cryopreservation, their centrifugation speed is generally 800-1000 rpm/min for 5-8 minutes at room temperature. Too high a speed will result in cell rupture and death.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Unique and unmatched
Creative Bioarray's cell products are unique and unmatched.
10 Sep 2023
Ease of use
After sales services
Value for money
Write your own review

