ONLINE INQUIRY

Human Calvarial Osteoblasts (HCO)
Cat.No.: CSC-7820W
Species: Human
Source: Bone
Cell Type: Osteoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human calvarial osteoblasts (HCO) stem from the tissue of the calvarial bone, the upper layer of the skull made up of flat plates of bone that surround key organs such as the brain. HCO cells essentially form and maintain the skull in normal physiological conditions. In the embryo, HCO cells play a central role in the initial structure of the skull: they make and release bones matrix elements, direct the deposits of calcium and phosphorus, and slowly construct the primitive structural architecture of the skull as a buffer for the brain. As individual grows, the brain and body expand indefinitely, HCO cells remain in operation and the skull can continue to expand, thicken and regenerate to meet physiological demands and changes in the mechanical world. As adults, despite the skull's reduced growth rate, HCO cells contribute to the microstructural remodeling and repair of the skull while its mechanical and metabolic equilibrium remain unchanged. For example, in the case of a small injury or mechanical stimulation every day to the skull, HCO cells are quickly able to initiate repair, making new bone matrix to fill in missing pieces, returning the skull to its original shape and functionality.
 Fig. 1. Human primary calvarial osteoblasts stained with TUNEL (Li Y, Sun S, et al., 2022).
Fig. 1. Human primary calvarial osteoblasts stained with TUNEL (Li Y, Sun S, et al., 2022).
E. faecalis Infection Triggered Apoptosis in Primary Osteoblasts
Chronic apical periodontitis (PAP) results in bone destruction because of an imbalance in bone turnover and development, typically with accelerated osteoblast apoptosis. One of the most significant microbes linked to PAP is Enterococcus faecalis, which causes osteoblast death. It is not clear whether it is involved in primary human osteoblast apoptosis, although the BCL-2 family is known to modulate apoptotic processes.
In this work, Li's team characterized the apoptotic activity of E. faecalis OG1RF in primary human calvarial osteoblasts in an attempt to discover targets for PAP. For measuring apoptosis in osteoblasts infected with E. faecalis OG1RF, they stained them with Annexin V-FITC/PI by flow cytometry (Fig. 1a). Apoptotic cells increased rapidly at 6 hours for a MOI of 500 and 1,000, and increased MOI-dependently at 12 hours (Fig. 1b). Early apoptosis reached a maximum at 6 hours with an MOI of 500, while late apoptosis emerged at MOIs of 500 and 1,000 at 6 and 12 hours respectively. JC-1 staining for mitochondrial membrane potential (ΔΨm) measurements indicated a significant decrease in (ΔΨm) over increasing MOIs, demonstrating intrinsic apoptosis activation (Fig. 2). For 12 hours, Caspase-3, 8/9 activity was high at MOI 1000 indicating that both intrinsic and extrinsic apoptotic pathways were involved (Fig. 3).
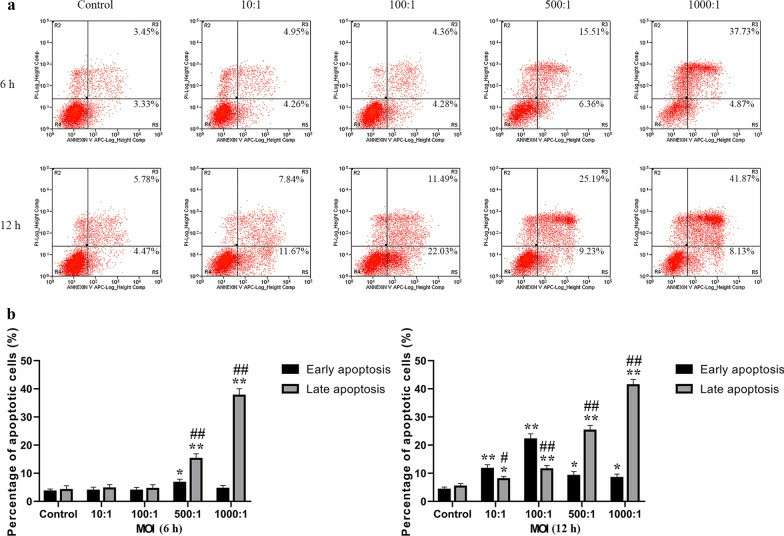 Fig. 1. Analysis of E. faecalis-induced apoptotic osteoblasts in early and late phases using Annexin V-FITC/PI staining (Li Y, Sun S, et al., 2022).
Fig. 1. Analysis of E. faecalis-induced apoptotic osteoblasts in early and late phases using Annexin V-FITC/PI staining (Li Y, Sun S, et al., 2022).
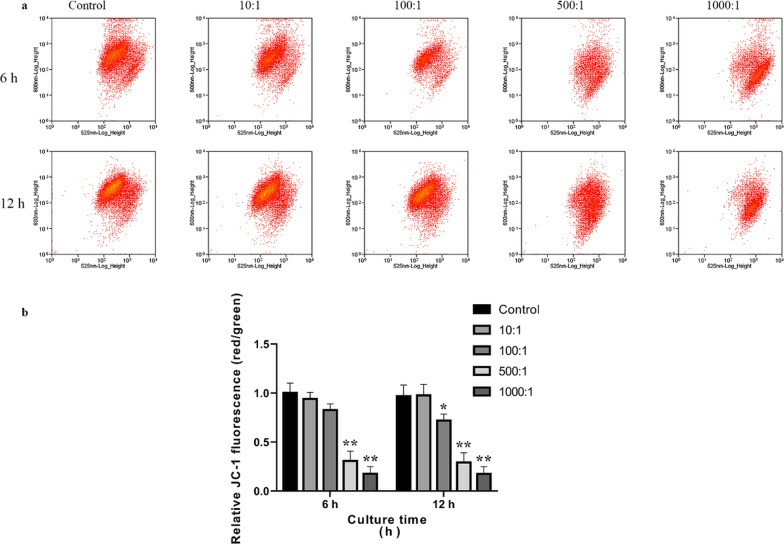 Fig. 2. Analysis of the ΔΨm in primary osteoblasts infected with E. faecalis OG1RF using JC-1 staining (Li Y, Sun S, et al., 2022).
Fig. 2. Analysis of the ΔΨm in primary osteoblasts infected with E. faecalis OG1RF using JC-1 staining (Li Y, Sun S, et al., 2022).
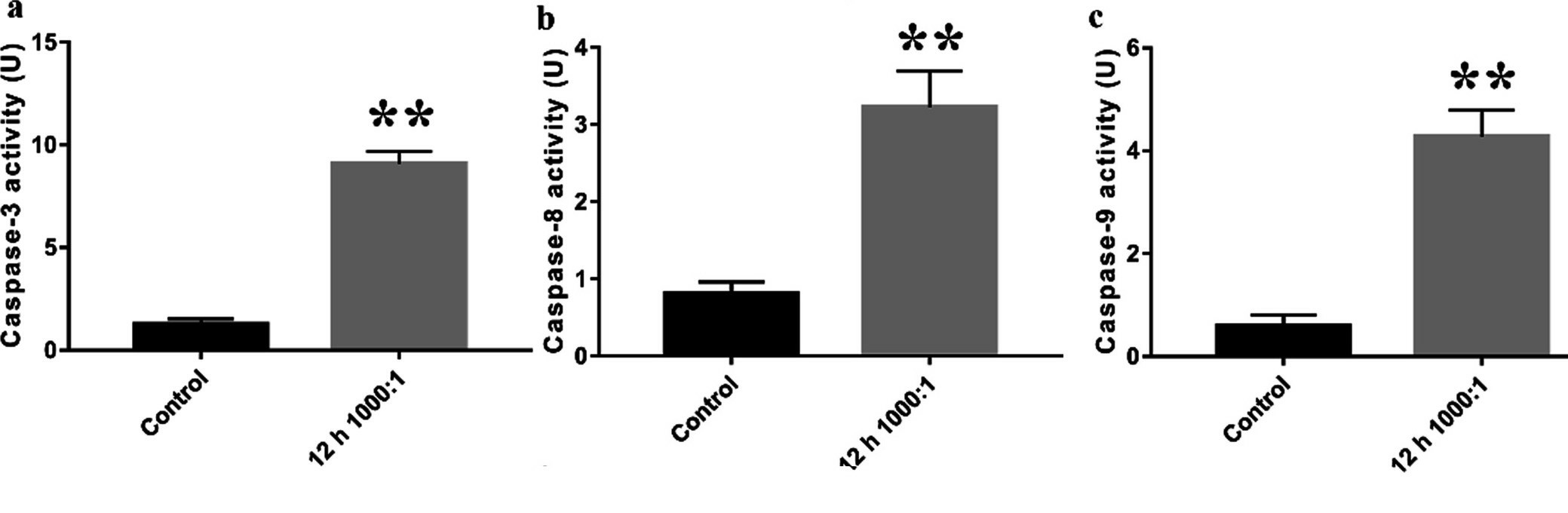 Fig. 3. Intrinsic and extrinsic apoptotic pathways were involved in E. faecalis-infected osteoblasts (Li Y, Sun S, et al., 2022).
Fig. 3. Intrinsic and extrinsic apoptotic pathways were involved in E. faecalis-infected osteoblasts (Li Y, Sun S, et al., 2022).
Effects of Equibiaxial Mechanical Stretch on Cell Viability in Human Calvarial Osteoblasts
Craniofacial sutures are essential for bone formation, and they can also carry mechanical stretch, which regulates bone development by acting on the sutures before they close. Orthodontists use mechanical stretch to repair craniofacial sutures. These remodeling activities involve osteoblast responses to mechanical stretching, such as differentiation, apoptosis and ECM production.
Zhang's team explored the effect of mechanical stretch on cell viability in human calvarial osteoblasts. Morphological alterations occurred in human calvarial osteoblasts (HCObs) after 12 hours of equibiaxial stretch. Light microscopy showed that the stretched cells expanded and shifted away from the force direction, but not the randomly placed control cells (Fig. 4). HCObs (area, perimeter, width, length) were bigger in the stretch-treated patients than in controls. At 12 and 24 hours, caspase 3/7 was significantly lower in stretch-treated HCObs than in controls (P < 0.05) (Fig. 5A). 24 hours stretching also reduced caspase 3/7 activity. The MTT results showed that 24 hours of stretching led to higher HCObs proliferation compared with control (Fig. 5B), more prolific at 24 hours than at 6 and 12 hours. CellTiter-Glo tests showed a significant increase in cell viability between stretch-treated HCObs and controls 6 and 24 hours later, which suggests that 2% lengthening at 0.2 Hz increases HCObs viability (Fig. 5C).
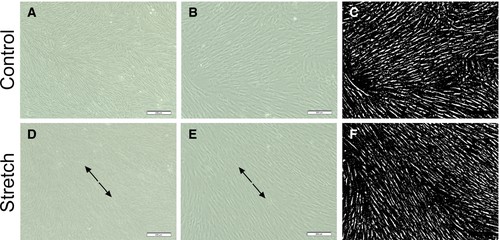 Fig. 4. Human calvarial osteoplasts (HCObs) exhibited altered morphology after an intermittent deformation of 2% for 5 s (0.2 Hz) every 60 s for 12 h (Zhang J, Xu S, et al., 2019).
Fig. 4. Human calvarial osteoplasts (HCObs) exhibited altered morphology after an intermittent deformation of 2% for 5 s (0.2 Hz) every 60 s for 12 h (Zhang J, Xu S, et al., 2019).
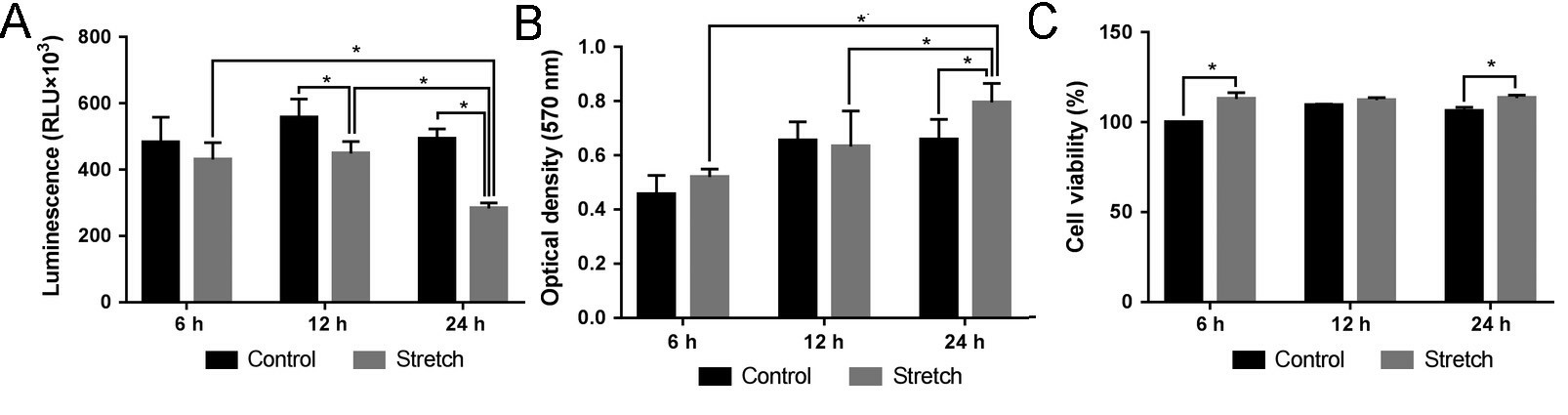 Fig. 5. (A) Effect of mechanical stretch on the activities of caspase 3/7. (B) Effect of mechanical stretch on cell proliferation. (C) Effect of mechanical stretch on cell viability (Zhang J, Xu S, et al., 2019).
Fig. 5. (A) Effect of mechanical stretch on the activities of caspase 3/7. (B) Effect of mechanical stretch on cell proliferation. (C) Effect of mechanical stretch on cell viability (Zhang J, Xu S, et al., 2019).
Creative Bioarray’s osteoblasts generally appear fibroblastic. They are cuboidal in lower densities and become spindle shaped as they become confluent.
It is not easy to give an exact number for the purity of osteoblasts as the tests used are all functional assays: alkaline phosphatase production and bone mineralization, which unless you have a synchronized culture, not all the cells are doing the same thing at the same time. Based on the cell morphology and other parameters, we consider the purity of the osteoblast culture to be approximately 95%.
It is recommended to use SuperCult® Osteoblast Growth Medium Kit (cat# CM-1149X) for the culturing of primary human osteoblasts.
You can either use chemical elution or digital image quantification. Based on Alizarin Red's fluorescence properties, fluorescence spectroscopy might work, as well. No matter which tool you are going to use, make sure that your pH is well calibrated. Otherwise your dye will change colour between yellow, red and purple.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Perfect
These cells are of high quality and allow reproducible results up to numerous passages.
09 July 2022
Ease of use
After sales services
Value for money
Write your own review

